Naming Molecular Compounds Worksheet - Chemistry Nomenclature

NAMING #5
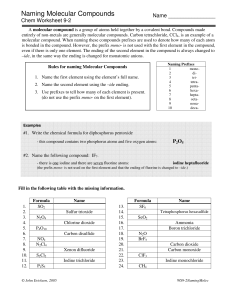
Naming Molecular Compounds
WHICH COMES FIRST?
The more positive of the 2 elements comes first. The more positive element is the one that is further to the left and down on the periodic table. (i.e., the less
______________________ one comes first
NAMING THE ELEMENTS
The more positive element is called by its name.
The more negative element is called by part of its name and the ending ide. These are the same names as the anions of the elements have.
e.g. oxide, sulfide, fluoride, chloride, bromide, iodide, nitride, hydride, selenide
If you are asked to write the name for this ionic compound- Al
2
S
3
- you just say
___________________________________
You do not need to say di or tri, because aluminum in a compound is always _____, and sulfur in an ionic compound is always ______, so they must exist in a __________ ratio.
However, in molecular compounds, the elements mostly don’t form
________________ charges, because the electrons are_______________, not lost or gained.
Example:
What is the formula for phosphorous chloride? The answer is:
__________________________________
There are 2 different compounds formed from phosphorous and chlorine. They are
PREFIXES MUST BE USED
You can not tell how many atoms of each element there is in a molecular compound (as you can by using the charges in ionic compounds). Therefore, you must put prefixes in the name to designate how many atoms of each element there is.
PCl
3
= phosphorous trichloride PCl
5
= phosphorous pentachloride
PREFIXES YOU MUST KNOW mono di tri tetra one two three four penta hexa hepta five six seven
IMPORTANT RULE:
The first element only has a prefix if there are more than one of those atoms.
The second element always has a prefix.
EXAMPLES – formulas to names
SF
6
CO
2
N
2
O
4
EXAMPLES – Name to formulas sulfur trioxide nitrogen trihydride diphosphorous pentasulfide
PRACTICE – Fill in the blanks
CCl
4
____________________________
Se
3
F
7
____________________________
SiS
2
____________________________
______________ carbon dioxide
______________ silicon tetrabromide
_______________ trinitrogen hexachloride