Acid or Base? Let the cabbage decide!
advertisement

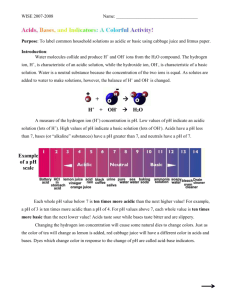
Acid or Base? Let the cabbage decide! Grade Level: 5 Strand: Understanding Matter and Energy Topic: Properties of and Changes in Matter Specific Expectations: - 3.7 Identify indicators of a chemical change (e.g., production of a gas, change in colour, formation of precipitate) Required Materials: - Jar of pickled red cabbage juice - Vinegar - Baking Soda - Lemon juice - Drinking glasses (3) Procedure: Part 1: Pour the red cabbage juice into a drinking glass. Place a small amount of baking soda into the cabbage juice, and observe the production of a blue-green foam. Pour several drops of vinegar onto the green foam, and observe the colour change from blue-green to pink. Add more baking soda to the solution, and observe the colour of the foam change back to blue-green. Part 2: In two new glasses, pour a small amount of red cabbage juice into each glass. In one of the glasses, pour a little vinegar into the cabbage juice. In the other glass, put a small amount of baking soda in with the cabbage juice. Pour a small amount of lemon juice into both glasses. Observe any changes in the glasses. Scientific Explanation: In this experiment, the colouring of the red cabbage juice acts as an acid/base indicator. There are compounds found in the cabbage, namely anthocyanins, that change colour depending on the pH of a solution. Anthocyanin is a pigment family found in many red or blue flowers, and fruits. The pH of a solution is linked to whether a solution is acidic or basic. If the solution has a pH lower than 7.0 it is considered an acid, and if it has a pH higher than 7.0 it is considered a base. Bases will turn the cabbage juice green, whereas acids will make the cabbage juice a deeper red. The following is a colour chart showing the colour that the red cabbage will turn when a substance of pH 1 through 12 is added. If a stronger base is added, one that has a pH of 13 or higher, the colour will actually change from green to yellow. References: http://nicholasacademy.com/scienceexperiment217cabbageindicator.html http://antoine.frostburg.edu/chem/senese/101/acidbase/faq/household-indicators.shtml The Ontario Curriculum Grades 1-8 Science and Technology Document (2007) Opportunities for other consideration: - For students sensitive to, or those that have an aversion towards, strong aromas, you may wish to replace vinegar with another acidic solution, maybe just the lemon juice, or even use a mask. - You could look for other clear liquids to test whether they are acidic or basic. - If enough base is added to the red cabbage, the colour can turn yellow. The class could make predictions as to how much they think will have to be added to attain this result, and then discuss why. - Discuss other acids and bases that the students know, and test out their pH levels. Glossary: pH – A measure of hydrogen ion concentration of a substance. The more hydrogen ions, the lower the pH value will be and the more hydrogen ions, the higher the pH value will be. A pH of 7.0 is considered neutral. Anthocyanin – A pigment family that gives many flowers, plants and fruits their blue or red colouring. Acid – A substance that, when in solution, releases hydrogen ions (H+). A substance with a pH lower than 7.0 is considered an acid. Base – A substance that, when in solution, produces hydroxide ions (OH-). A substance with a pH higher than 7.0 is considered an acid. Indicator – A substance that displays an easily observable change when conditions in its solution change. Chemical Change – Dissociation, recombination of rearrangement of atoms is evidence of a chemical change. Michelle Ponert and Elissa Zohar