Name ______ Date Period ______ RED CABBAGE AS AN ACID

Name _____________________________________ Date ______________________ Period _______
RED CABBAGE AS AN ACID-BASE INDICATOR
PURPOSE:
The purpose of this lab is to use red cabbage juice to determine the pH of common household substances both quantitatively and qualitatively.
INTRODUCTION:
Acids are substances that donate hydrogen ions (H + ) in solution. Bases are substances that accept protons and/or release hydroxide ions (OH ) in solution. Buffers are solutions that help prevent major changes or swings in pH. Buffers, circulating throughout the human body, are extremely important in keeping your blood (range of 6.5-7.5) at a pH of around 7.4. Major swings in pH can disrupt chemical reactions in the body.
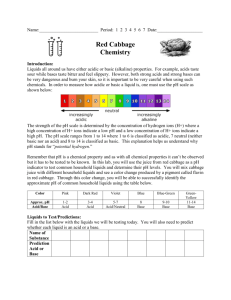
Red cabbage contains a pigment molecule called flavin (an anthocyanin). This water-soluble pigment is also found in apple skin, plums, poppies, cornflowers, and grapes. Very acidic solutions will turn anthocyanin a red color. Neutral solutions result in a purplish color. Basic solutions appear in greenish-yellow. Therefore, it is possible to determine the pH of a solution based on the color it turns the anthocyanin pigments in red cabbage juice. The color of the juice changes in response to changes in its hydrogen ion concentration.
MATERIALS:
Pre-filled droppers with household items to test.
Test Tubes with cabbage extract.
LAB SAFETY:
YOU MUST WEAR SAFETY GOGGLES AT ALL TIMES DURING THE LAB. ASSUME THAT
ALL OF THE SUBSTANCES YOU ARE WORKING WITH ARE DANGEROUS.
PROCEDURE:
1.
Using the data table below, predict the pH values for the household items your are testing.
Record the starting color of the cabbage extract.
2.
Each well contains a different substance. Make sure you know what you are testing. Use the board as a reference.
3.
Each test tube contains cabbage juice. Use the prefilled droppers to add drops (one at a time) of each household substance to the appropriate test tube. STOP WHEN YOU SEE A
COLOR CHANGE.
4.
Observe and record any color changes that you observe.
5.
Use the approximated pH table (below) to determine if your household item is an acid, basic or is neutral.
6.
When you are finished, please rinse and dry your test tubes.
DATA TABLE:
Table 1.0 Red Cabbage Color at Various pH Levels
Approximate pH 2 4 6 8 10 12
Color of Extract Red Red-Violet Violet Blue Blue-Green Green
Table 1.1 pH Values for Common Household Items Using Red Cabbage Extract
Household Item Predicted
Tap Water pH
Starting
Color
Color
Change
Approximate pH
White Vinegar
Bleach
Rainwater
Lime-Away
Soda
POST LAB QUESTIONS:
Acid, Base, or
Neutral
1. In pure water, about 1 water molecule in 550 million splits to form ions. Show the summary of the
chemical equation below:
2. Define acid. Give an example.
3. Define base. Give an example.
4. What does the pH scale measure? Explain.
5. How many times greater is the hydrogen ion concentration if we move from a pH of 2 to a pH of 1?
Why is lemon juice far less dangerous (pH of 2) than hydrochloric acid (with pH of 1)?
6. Why are strong acids and bases dangerous?
7. What are buffers and why are they important to the human body? What is the pH of human blood?