a. ionic bonds
advertisement

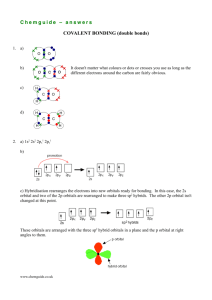
BONDING A. IONIC BONDS Ionic bond – electrostatic attraction between two oppositely charged ions + _ cation + anion + or _ metal + nonmetal or Very strong bond Involves a transfer of electrons Results in the formation of salts (or group 1A hydroxides) + _ metal + polyatomic ion (All salts are ionic compounds but not all ionic compounds are salts.) Ionic compound – compound composed of positive and negative ions Ex: Na + Cl Na+Cl- Na + Cl + Na Cl + Na Cl Characteristics of Ionic Compounds: Crystalline solids at room temperature Have high melting points (MP indicates bond strength) Tend to dissolve in water Conduct electricity in the molten state Look at the reaction of Na + Cl: 1. Nao + ionization energy Na+1 + 1 e- ENDO (bonds breaking requires energy) 2. Clo + 1e- Cl- + electron affinity energy EXO (bonds forming releases energy) 3. Na+1 + Cl- NaCl + lattice energy EXO (bonds forming releases energy) Lattice energy – the energy given off when two oppositely charged ions bond to form a crystal lattice or solid salt. Octet Rule – atoms react to achieve the electron configuration of a noble gas One endothermic step and two exothermic steps results in a NET EXOTHERMIC process. Compounds form to achieve the lowest possible energy. Ionic Crystal Structure of Compounds NaCl crystal: In the 3 D structure of a NaCl crystal, each ion is surrounded by 6 oppositely charged ions.* There are, therefore, many strong ionic bonds between all adjacent oppositely charged ions. These large attractive forces result in a stable structure with a very high melting point. A crystal’s arrangement is a regular repeating pattern called a lattice. the shape of a crystal depends on the internal arrangement of the ions (or atoms) the many possible crystal arrangements of ions depends on their sizes Coordination number – the number of ions of opposite charge that surround each ion in a crystal *the coordination number of NaCl is 6 B. METALLIC BONDS Metallic bonds – attractions of free-floating valence electrons for positively charged metal ions - may form between atoms of the same metal or atoms of different metals. Alloys – solutions of different metals made by melting metals together and cooling them Atoms that form metallic bonds must have: vacant valence orbitals that can hold additional electrons low ionization energies so that loosely held electrons are available for bonding Atoms in metals are packed in compact crystal forms that are most commonly cubic in shape. Characteristics of Metals: malleable and ductile good conductors of electricity C. COVALENT BONDS Covalent bond – mutual attraction of different nuclei to the same electron’s orbital - occurs between nonmetal atoms Very strong bond Involves a sharing of electrons Results in the formation of molecular compounds Molecular compound – compound composed of nonmetals held together by covalent bonds Ex: Cl + F Cl F Cl + F ClF Cl F Characteristics of Molecular Compounds: Tend to be liquids or gases at room temperature (though some are solids) Have low melting points Contain 2 types of forces: 1. intramolecular forces – strong covalent bonds within the molecules 2. intermolecular forces – weaker attractive forces between the molecules Ex: Water molecules in an ice crystal: + INTRAMOLECULAR: Strong covalent bonds in which electrons are shared by the O and H _ + + _ + INTERMOLECULAR: The positive end of one H2O mc is attracted to the negative end of an adjacent H2O molecule. _ + + Diatomic Molecule – molecule composed of 2 atoms (this includes all HONClBrIF’s) Both nuclei attract the same Look at H2 : electrons Both atoms strive H 1s1 x H 1s1 to fill their 1s orbital so both H’s attract the pair of bonding electrons + + H–H Shape of H2 is linear + + atomic orbitals overlap to form new molecular orbital s to s bonding is non-directional because s orbitals can approach each other from any direction and overlap to form a bond Look at F2 : F 2pz is where bonding occurs py py x 1s2 2s2 2px2y2z1 x F x F + pz py new molecular orbital forms + pz x F 1s2 2s2 2px2y2z1 2pz orbitals overlap so now both atoms achieve a stable octet py F–F Shape of F2 is linear directional bonds - bonding in which atom’s orbitals must approach at a specific direction in order for the orbitals to overlap to form a bond (s to p, p to p, s to d, & p to d) non-polar covalent bond – covalent bond in which there is an equal attraction for the shared electrons All bonds in HONClBrIFs are nonpolar (purely) covalent bonds where there is an equal sharing of electrons. electronegativity – a number that indicates the relative attraction an atom has for a shared pair of electrons in a bond; the higher the number, the greater the attraction electronegativity difference ( EN) is an indication of the type of bond formed: electronegativity difference 0 – .3 = nonpolar covalent bond (equal sharing of electrons) electronegativity difference .4 – 1.6 = polar covalent bond (unequal sharing of electrons) electronegativity difference (transfer of electrons) > 1.7 = ionic bond polar covalent bond – a covalent bond between 2 atoms in which one atom has a greater pull (attraction) on the shared electrons (unequal sharing) Look at H2O : O _ .. x O 1s2 2s2 2px2y1z1 + H x O x H O + : + H H H 1s1 1 H’s 1s overlaps the O’s 2px lobe and the H2O mc’s shape is bent b/c the + H bonds are polar b/c O has H 1s1 other H’s 1s overlaps the O’s 2pz lobe y and z axes are perpendicular a greater pull on e- than H O = 3.5; H = 2.1 EN = 1.4) H MOLECULAR POLARITY polar molecule – a molecule in which one region of the molecule has a higher electron density causing the molecule to have positive and negative regions or ends. The polarity of a molecule is determined by the following: the polarity of the bonds the overall distribution of the valence electrons the symmetry/shape of the molecule Compare the following molecules: Methane CH4 H H C H H Ammonia Water NH3 H2O _ -- H N H H H O H Bonds: C - H C = 2.5 H = 2.1 EN = .4 Bonds: N - H N = 3.0 H = 2.1 EN = .9 Bonds: O - H O = 3.5 H = 2.1 EN = 1.4 bonds are slightly polar bonds are moderately polar bonds are very polar shape is tetrahedral shape is pyramidal shape is bent molecule is non-polar molecule is moderately polar molecule is very polar b/c mc is symmetrical b/c mc is asymmetrical b/c mc is asymmetrical and has 1 exposed e- pair and has 2 exposed e- pairs gas at room temperature liquid that evaporates easily liquid at room temp (no attractive forces b/t mc) (weak intermolecular forces) (strong intermolecular forces) low boiling point high boiling point MOLECULAR GEOMETRY VSEPR (valence shell electron pair repulsion) Theory – because electron pairs repel, molecules adjust their shape so that the valance (outer shell) electron pairs are as far apart as possible. Shape Bond Angle Linear 180o Example .. .. : F: F: .. Bent .. H:O : 105o F2 .. H2O .. H (The asymmetric water molecule has 2 exposed (unshared) electron pairs on one side of the molecule. Due to their repulsion of like charges, they require more space and consequently decrease the angle between the two hydrogen atoms.) Trigonal Planar 120o F .. B F Pyramidal 107o BF3 F .. H : N : H NH3 .. H Tetrahedral 109.5o H .. H : C : H .. H CH4 POLYATOMIC IONS Polyatomic ion – a group of atoms that act as a unit and carry a charge Made up of nonmetals Bonds WITHIN polyatomic ions are covalent bonds Cannot exist independently (can only exist as part of a compound) Examples: Hydroxide OH-1 O H Hydronium Ammonium H3O+1 NH4+1 H O H H +1 H H N +1 H H +1 [ O - H ] -1 H–O–H H (linear) H H-N-H H +1 (pyramidal) (tetrahedral) Sulfite Sulfate Phosphate SO3-2 SO4-2 PO4-3 O O S O -2 O O S O O O O O S O -2 (pyramidal) O -2 O O S O O (tetrahedral) P -3 O O -2 O O P O O -3 (tetrahedral) Coordinate covalent bond – a covalent bond in which one atom contributes both electrons in the shared pair MOLECULAR ORBITALS (orbitals of molecules) Molecular orbitals are the result of the overlap of atomic orbitals when 2 atoms form a bond. Types of molecular orbitals: 1. sigma bond – bond formed when 2 atomic orbitals combine to form a molecular orbital that is symmetrical along the axis connecting 2 nuclei END-TO-END orbital overlap is extensive therefore bonds are very strong Two “s” atomic orbitals can overlap to form a sigma-bond molecular orbital: + s atomic orbital + s atomic orbital sigma-bonding molecular orbital Two “p” atomic orbitals can overlap head to head to form a sigma-bonding molecular orbital: + p atomic orbital + p atomic orbital sigma-bonding molecular orbital 2. pi bond - bond formed when 2 atomic orbitals combine to form a molecular orbital in which the bonding electrons are located in sausage shaped regions above and below the bond axis SIDE-TO-SIDE orbital overlap is not as extensive (as in a sigma bond) therefore the bonds tend to be weaker Two “p” atomic orbitals can overlap side-to-side to form a pi-bonding molecular orbital: + p atomic orbital + p atomic orbital pi-bonding molecular orbital ORBITAL HYBRIDIZATION Hybridization – the combining of 2 or more orbitals of nearly the same energy into orbitals of equal energy occurs when atoms promote electrons into nearby orbitals to increase their bonding capacity (occurs in atoms of Gr. 2, 3, 4 or 6A) occurs when several atomic orbitals mix to form the same number of hybrid orbitals hybrid orbitals are equivalent because they have the same size, shape and energy all sigma bonds and lone pair electrons (exposed or nonbonding e-) require hybrid orbitals Look at BeH2: Beryllium promotes one of its2s electrons to its empty 2p orbital Be’s one 2s orbital and one 2p orbitals then mix to form two sp hybrid orbitals 2s 2p hybrid sp orbitals Be mix to form unhybridized 2p orbitals and Now the 1s orbitals of the Hydrogen atoms can overlap with the sp hybrid orbitals of the Be: sp The resulting molecule is linear: H – Be – H Be because the sp hybrid orbitals form 180o angles H H s H atomic orbital and one hydrogen overlaps on each end. s Be hybrid orbitals H atomic orbital Bonding Beryllium Hydride molecule The H – Be bonds are sigma bonds because they are the result of the end-to-end orbital overlap. Hybridization of Carbon - Carbon promotes one of its 2s electrons to its empty 2p orbital in order to s p p p increase its bonding capacity. Look at Methane: When methane forms – Carbon’s one 2s orbital and three 2p orbitals mix to ( CH4 ) s form four sp3 hybrid orbitals to provide four bonding sites for the four H atoms. p p p C: s s s s sp3 H’s: H + Hydrogen atomic orbitals H H H + Carbon hybrid orbitals Hydrogen atomic orbitals Methane molecule All four bonds formed are sigma bonds because they are the result of end-to-end orbital overlap. The shape of the methane molecule is tetrahedral because the sp3 bond angles are 109.5o Look at Ammonia (NH3) : s p p p N When H bonds with N, four sp3 molecular orbitals must form: Three of the orbitals provide bonding sites for the H’s H’s H H H One of the orbitals provides a site for the lone pair of e.. Not all of the hybrid orbitals of the central atom must be used for bonding, N H That is, lone pair electrons can also be accommodated in hybrid orbitals. H H The 3 bonds formed are sigma bonds because they are the result of end-to-end orbital overlap. The sp3 bond angles here are 107o, because the bonding electrons are repeled by the unshared (lone) pair of electrons and require more room, therefore the molecular shape is pyramidal. MULTIPLE BONDS Look at Ethene: ( C2H4 ) : Each carbon will form two C – H single bonds and one C = C double bond H C H single bond - 1 shared pair of electrons H double bond - 2 shared pairs of electrons C H In order for each Carbon to bond to three atoms: One s and two p orbitals of each C form three sp2 hybrid bonding orbitals s p p p Each Carbon’s additional p orbital remains unhybridized H O H O C O (C) T Two of the sp2 orbitals of each C overlap end-to-end with an s orbital of H to form a sigma bond. The other sp2 orbital of each Carbon overlaps end-to-end to form a C – C sigma bond.* The unhybridized p atomic orbital of each Carbon overlap side-to-side to form a C – C pi bond.* *The C = C double bond is comprised of 1 sigma bond and 1 pi bond. As a result of the sp2 orbital bonding, the H – C – H bond angles are 120o. Hydrogen atomic orbitals Carbon atomic orbitals and hybrid orbitals Hydrogen atomic orbitals Ethene molecule Look at Ethyne ( C2H2 ) : Each carbon will form one C – H single bond and one C H C C H = C triple bond triple bond - 3 shared pairs of electrons In order for each Carbon to bond to two atoms: One s and one p orbital of each C form two sp hybrid bonding orbitals s p p p Each Carbon’s additional two p orbitals remain unhybridized H O C O (C) (C) T T One of the sp orbitals of each C overlap end-to-end with an s orbital of H to form a sigma bond. The other sp orbital of each Carbon overlaps end-to-end to form a C – C sigma bond.* The unhybridized p atomic orbitals of each Carbon overlap side-to-side to form two C – C pi bonds.* *The C = C triple bond is comprised of 1 sigma bond and 2 pi bond. As a result of the sp orbital bonding, the H – C – H bond angles are 180o and the shape of the molecule is linear. Hydrogen atomic orbitals Carbon atomic orbitals and hybrid orbitals There are 3 types of hybrid orbitals: Hydrogen atomic orbitals Ethyne molecule To determine the type of hybrid orbitals formed: sp – comprised of 2 orbitals 1. Count the # of atoms bonded (to the central atom) or count the number of sigma bonds sp2 – comprised of 3 orbitals 2. Count the number of non-bonding electron pairs sp3 – comprised of 4 orbitals 3. Sum 1 + 2 above to find # of hybrid orbitals 4. Match # of hybrid orbitals to type (see bullets at left) RESONANCE the extremely rapid shifting of the bonding of a molecule between 2 or more stable arrangements 2 or more equally valid electron dot structures can be drawn for a molecule the bonds are considered a mix or hybrid of the extremes makes a molecule more stable Ozone O3 .. O O O O Sulfur Trioxide O O O O O O O SO3 O .. S O O O O O O O S S S O O O Benzene C6H6 Or O O