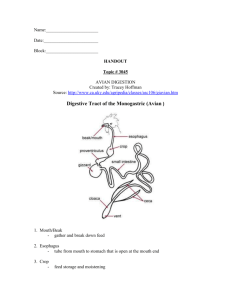
Basic function of the alimentary tract:
advertisement

Basic function of the alimentary tract: providing the nutrients needed by the body - energy substrates - essential macronutrients :essential AA essential FA - essential micronutrients : vitamines trace elements - water, electrolytes Digestion: Physiological point of view: 1. movement of food through the alimentary tract 2. secretion of the digestive juices 3. absorption Biochemical point of view: To digest food means to brake down large polymers to monomers before they can be absorbed. Our program: 1. general considerations about absorption and intestinal epithelial transport 2. digestion and absorption of proteins 3. digestion and absorption of carbohydrates 4. digestion and absorption of triacylglycerols and cholesterol 5. structure, function and metabolism of lipoproteins 6. bile acid metabolism 7. absorption of vitamines , metabolism of iron 8. review of functions of individual parts of alimentary tract Epithelial transport and principles of absorption Basic mechanisms of absorption: - diffusion (movement along electrochemical gradient). May be passive or facilited. - secondary active transport (coupled with Na) Energy gives NaK-ATPase of contraluminal surface. - both ways: Na-cotransport through luminal membrane of enterocyte and diffusion through the contraluminal one Water is absorbed by the process of osmosis – the chyme remains isoosmotic. Water follows absorbed substances into the blood. Absorption of sodium ions: !crucial for energization of nutrient uptake and water absorption! - active component: NaK-ATPase of contraluminar surface - passive component: various ways how to move sodium from the lumen of intestine to the cytoplasm of enterocyte: 1. coupled transport with monosaccharides, AA, bile acids 2. Na/H exchange (plus Cl/HCO exchange) 3. Electrogenic Na chanels (lower portion of large intestine, regulated by aldosterone) Chloride ions follow sodium along electrical gradient (passive diffusion). Cl/HCO exchanger also exists. Digestion and absorbtion of proteins Whole proteins and oligopeptides are not absorbed (with the exception of a short period after birth). Enzymes participating in splitting a peptide linkage: 1. endopeptidases = proteases: attack internal bonds and liberate large peptide fragments, di- and tripeptides 2. exopeptidases: cleave off one AA from the –COOH (karboxypeptidases) or –NH3 (aminopeptidases) terminus Gastric digestion – pepsin Pepsin is capable of digesting essentialy all the types of protein, major products of its action are large peptide fragments. Secreted as zymogen (pepsinogen), autoactivationautocatalysis. Favorable pH ranges around 2-3 active form present in the stomach only. Prefers peptide bonds formed by aromatic AA (Phe, Tyr) The role of gastric HCl: 1. denaturation of proteins 2. make pH optimal for the action of pepsin Enzymes of pancreatic juice ENZYME Trypsin Chymotrypsin Elastase Carboxypeptidase A Carboxypeptidase B PROENZYME Trypsinogen ACTIVATOR AA prefered Enteropeptidase Arg, Lys , trypsin Chymotrypsino- Trypsin Tyr, Trp, Phe, gen Met, Leu Proelastase Trypsin Ala, Gly, Ser Procarboxypepti Trypsin Val, Leu, Ile dase A Procarboxypepti Trypsin Arg, Lys dase B Other names: Serine proteases: in the active site of the enzyme is serine group: trypsin, chymotrypsin, elastase Zn-peptidases: metalloenzymes: pancreatic carboxypeptidases A and B (Digestion and absorption of proteins – continued) Intestinal phase of protein digestion The combined action of pancreatic and gastric endopeptidases and pancreatic exopeptidases (carboxypeptidase A, B) results in the formation of mixture of free AA (40%) and small polypeptides (60%) Enzymes of the small intestinal surface ENZYME Aminopeptidase A Aminopeptidase N Dipeptidyl aminopeptidase Leucine aminopeptidase Endopeptidase 24.11 Enteropeptidase SUBSTRATE Acidic AA Neutral AA NH2terminus Pro, Ala Neutral AA of oligopeptide Protein(!), internal hydrophobic AA Trypsinogen COMMENTARY Present as active form, need not activator! Differs in function! Free amino acids , di- and tripeptides are absorbed, the cleavaging to amino acids is finished within the cytosole of the enterocyte! Carrier – mediated transport of free amino acids secondary active transport: against concentration gradient ( cotransport with Na) in the luminal membrane, Na independent in the contraluminal membrane transporters are stereospecific, L- forms of AA are absorbed only There are six transport systems: 1. for acidic AA: Asp, Glu 2. for basic AA: Lys, Arg (and cystine) 3. for neutral AA with short or polar side chain: Ser, Thr, Ala 4. for neutral AA with aromatic or hydrophobic side chain: Phe, Tyr, Met, Val, Leu, Ile 5. for imino AA: Pro, Hyp 6. for - AA: taurin, -Ala Di- and tripeptides are absorbed via specific transport system and hydrolysed to free AA within the cytosol of enterocyte. Digestion and absorption of carbohydrates Dietary carbohydrates involve mono-, di- and polysaccharides. Essentially all the carbohydrates are absorbed in the form of monosaccharides: di- and polysaccharides recquire hydrolysis. 1. - amylase the most important enzyme for starch and glycogen cleavaging. present in saliva (ptyalin) and pancreatic juice specifity for internal -1,4 bonds (-1,6 bonds are not attacked, neither -1,4 bonds that serve as branch point) hydratation of starch (during heating) is essential for efficient action of this enzyme substrates: -1,4 glucose polymers (starch and glycogen). Other polysaccharides cannot be hydrolysed nor absorbed! products: maltose, maltotriose, - limit dextrines (oligosaccharide with average 8 Glc units and one or more -1,6 branching) 2. Surface enzymes: oligo- and disaccharidases - enzymes present on the luminal plazma membrane of the enterocyte - for final hydrolysis of di- and oligosaccharides ( from the diet or made by the action of -amylase) - clip off one monosaccharide unit from non-reducing end of oligosaccharide ( or cleave disaccharide) SPECIFITY SUBSTRATE ENZYME (bond to hydrolyze) (common name) Amylose Glucoamylase Glc-(14) Glc (of oligo- or polysaccharide) Glc-(14) Glc Glc-( 16) Glc Glc-(11) Glc Glc-(11) Fru Gal-(14) Glc Maltose, maltotriose Isomaltose,- dextrine Trehalose Succrose Lactose Maltase Isomaltase Trehalase Succrase Lactase Carbohydrates that are not hydrolyzed by the action of -amylase and/or surface enzymes cannot be absorbed are anaerobically metabolised by the intestinal bacteria can cause diarrhea . Absorbtion of monosaccharides D-glucose and D-galactose are absorbed by Na – coupled carrier – mediated transport, D-fructose by facilitated diffusion. Other monosaccharides (e.g. pentoses, L-forms etc.) are absorbed only in very small quantities by passive diffusion. Transport of glucose and galactose - SGLT – 1 : Na – dependent transporter on the luminal surface of enterocyte - GLUT-2 : transporter on the contraluminal plazma membrane of enterocyte ( Na – independent, facilitated diffusion) This way of moving glucose through a cell is very similar in kidney (uptake of glucose in proximal tubular cell) Transport of fructose is slightly different from that of most other monosaccharides. Because fructose becomes rapidly phosphorylated within the epitelial cell and than converse to glucose, cytosolic concentration of fructose remains low. Therefore, fructose is transported by facilitated diffusion, using a specific carrier protein. Digestion and absorbtion of lipids Lipids are defined by their poor water solubility. In aqueous phase they tend to aggregate to large complexes and become not easily accessible to the digestive enzymes and to the cell surface for absorbtion. Therefore, physical changes (solubilization) must be connected to chemical changes (hydrolysis) during digestion and absorbtion. Stomach fase of lipid digestion – acid-stable lipase - the enzyme is secerned by Ebner‘s glands at the back of the tongue and small amounts in the stomach - initial hydrolysis of non-emulgated TAG - products of reaction – MAG and FFA – spontaneously adsorb to water/lipid interface and increase the surface area (dispersion into smaller droplets) Pancreatic lipase – the most important enzyme for TAG hydrolysis - specifity: for esters in the position 1 and 3 - products: FFA and MAG - secerned as active form, but strongly inhibited by the bile acids - activated: by the action of colipase Colipase binds to both the surface of lipid droplets and to the lipase, thereby anchoring and activating of the enzyme. Colipase is secreted as proenzyme and is activated by the action of trypsin. The function of bile acids - biological detergents secerned by the liver - in sufficent concentration (2-5 mM) form aggregates called micelles schema micely Bile salt micelles can solubilize other lipids (MAG, FFA, PL,C) forming so-called mixed micelles. The products of TAG hydrolysis are continuously transferred from the emulsion droplets to the micelles. When the mixed micelle is close to the cell surface, absorbtion can occure. MAG and FFA are absorbed by the proces of passive diffusion. Reesterification of MAG and FFA within the enterocyte is responsible for keeping the concentration gradient. Digestion of other lipids: - lipid esterase: non-specific, attacks MAG, CE, etc. - cholesterolesterhydrolase - phospholipase A2 Resythesis of TAG, formation of chylomicrons Fatty acid with short and medium chain (12 C) pass through the enterocyte directly into the portal blood. Long-chain fatty acids are resynthesizet into TAG. Resynthesized TAG with phospolipids and Apo B48 and Apo AI form chylomicrons. They are released into the lymph. Schema Devlin1082hore Structure and metabolism of lipoproteins Basic function: transport of water insoluble material – TAG and cholesterol in the aqueous environment – blood. General structure: Schéma lipopr. Types of apoproteins: Function of Apo Type of Apo Apo AI Cofactors Apo AII Apo CII Ligands for Apo AI lipoprotein receptors Apo B100.E Apo E Structural apoproteins Apo AI Apo B48 Apo B100 Commentary For LCAT For hepatic lipase For LPL For HDL receptors For IDL and LDL For chylomicron remnants HDL Chylomicrones VLDL, IDL, LDL Review of functions of various parts of alimentary tract Enzymes are necessary for digestion. Where do they act? 1. the lumen of alimentary tract – enzymes from glands - - - salivary glands: amylase starts cleavaging starch and glycogene stomach: pepsin= endopeptidase splitting almost any types of ingested proteins gastric lipase is responsible for digesting 30% of ingested lipids pancreas: proteolytic enzymes: trypsin, chymotrypsin, carboxyypeptidase -amylase pancreatic lipase and cholesterolesterase other: ribonuklease, deoxyribonuklease liver secretes bile (no enzymes!): bile acids 2. luminal plazma membrane of enterocyte – brush border. Substrates for these enzymes are the nutrient oligomers and dimers that result from the pancreatic digestion of food. - disacharidases: maltase, sucrase/isomaltase, glucoamylase, lactase, -glucosidase - dipeptidases and aminopeptidases: many types very specific for hydrolyzing individual types of peptide linkage - other: enteropeptidase, alcaline phosphatase 3.cytoplasm of enterocyte: - hydrolysis of absorbed di- and tripeptides (60% of ingested proteins is absorbed this way) - triglyceride resynthesis, formation of chylomicrones