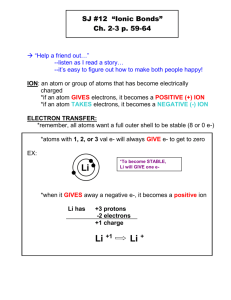
Common Ions Based on Groups
advertisement

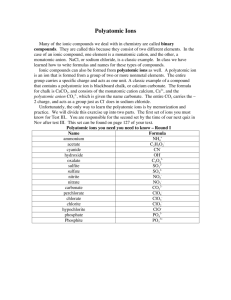
Name: ______________________________ Per: _____ Ch. 5 Enrichment ~ Polyatomic Ions Reading and Questions ~ So far, we have studied that ions are single atoms that have lost or gained electrons in order to become stable. Yet, in nature, many ionic compounds contain polyatomic ions, which are ions made up or more than one atom. The atoms within a polyatomic ion are usually very tightly bound together, so the ion retains its identity within ionic compounds and over the course of many chemical reactions (the atoms do not easily separate). The table below lists the formulas and the charges for several polyatomic ions. The charge given to a polyatomic ion applies to the entire group of atoms. Although an ionic compound containing one or more polyatomic ions contains more than two atoms, the polyatomic ion acts as an individual ion. Therefore, the chemical formula for the compound can be written following the same rules used for a compound containing only two ions. Because a polyatomic ion exists as a unit, never change the subscripts of the atoms within the ion. If more than one polyatomic ion is needed, place parentheses around the ion and write the appropriate subscript outside the parentheses. For example, the formula for magnesium chlorate is Mg(ClO3)2. Note that the ammonium ion is the only common polyatomic positive ion (cation). How can you determine the formula unit for an ionic compound containing a polyatomic ion? Let’s consider the compound formed from the ammonium ion and the chloride ion. NH4+ (ammonium ion) Cl- (chloride ion) Because the sum of the charges on the ions is zero, the ions are in a one-to-one ratio. The correct formula unit for this compound is NH4Cl. Group (or column) 1 2 15 16 17 Common Ions Based on Groups Atoms that commonly form ions Charge on ions H, Li, Na, K, Rb, Cs Be, Mg, Ca, Sr, Ba N, P, As O, S, Se, Te F, Cl, Br, I 1+ 2+ 321- 1) Look at the chart “Common Ions Based on Groups.” Why do the atoms in group 1 form a positive ion? _________________________________________________________________________________ 2) Using the same chart, consider: Why do the atoms in group 15 form ions that have a 3- charge? _________________________________________________________________________________ In ionic compounds, the charges of each ion need to be balanced in such a way as to have the overall charge of zero. For instance, Na+ and Cl- The +1 and -1 charge cancel each other out and the net charge of the ionic compound is zero. K+ and S2- Since potassium and sulfur do not have equal, opposite charges, another potassium must be added in order to make the charge of the ionic compound zero (neutral). Therefore, the ionic compound formula is K2S. 3) Look at the list of the common polyatomic ions (on the previous page) and write the formulas for the following elements: Ions Charges of ions/polyatomic ion Ionic Compound Formula + Sodium and hydroxide Na and OH NaOH Lithium and nitrite Hydrogen and cyanide Sodium and perchlorate Hydrogen and sulfate Magnesium and nitrite Barium and chlorate Hydrogen and acetate Strontium and peroxide Cesium and sulfite Hydrogen and arsenate Barium and phosphate Aluminum and chromate Potassium and chromate Magnesium and carbonate Calcium and chlorate 4) In 2-3 sentences, summarize what you learned from this reading and exercise: __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________