Review Questions – Chemical Names and Formulas
advertisement

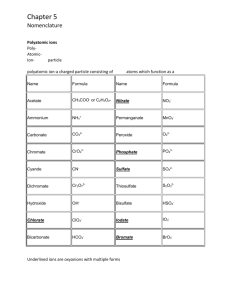
Review Questions – Chemical Names and Formulas Chapter 9 1. Consists of a single atom with a positive or negative charge. 2. A postively charged ion. 3. What is the charge on ions formed from the alkali metal group? 4. What is the formula and charge for the aluminum ion? 5. A negatively charged ion? 6. What is the charge on the ions formed from the halogen family? 7. Write the formula and charge for the nitride ion. 8. The ions that are composed of more than one atom are called? 9. Which does not have a -2 charge? sulfite, dichromate, cyanide, or silicate. 10. What is the formula and charge for the permanganate ion? 11. What is the formula and charge for the phosphite ion? 12. Which two polyatomic ions end in ide? 13. Which polyatomic ion is the only cation? 14. Binary means the compound is made of ______ elements. 15. Binary ionic compounds are made of what two type of elements? 16. What is the correct formula for tin (II) fluoride? 17. What is the correct formula for aluminum oxide? 18. What is the correct formula for calcium phosphate? 19. What is the correct formula for ammonium sulfate? 20. What is the correct formula for chromium (III) nitirite? 21. Name the following binary ionic compounds: 1. NaF 2. Mg(NO3)2 3. Fe2(C2O4)3 22. What is the prefix used for the number 6 to name molecular compounds? 23. What is the formula for sulfur trioxide? 24. What is the formula for tetraphosphorus decaoxide? 25. Write the names for the following molecular compounds 1. N2O5 2. Cl2O8 3. ClF3 26. What is the formula for nitric acid? 27. What is the formula for hydrochloric acid? 28. What is the law that states "whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers". 29. Compounds made from two nonmetals. 30. The law that states that in samples of any chemical compound, the masses of the elements are always in the same proportions. ANSWERS: 1. monatomic ion 2. cation 3. 1+ 4. Al3+ 5. anion 6. 17. N38. polyatomic ion 9. cyanide 10. MnO411. PO3312. cyanide and hydroxide 13. ammonium 14. 2 15. metal and a nonmetal 16. SnF2 17. Al2O3 18. Ca3(PO4)2 19. (NH4)2SO4 20. Cr(NO2)3 21. sodium fluoride, magnesium nitrate, iron (III) oxalate 22. hexa 23. SO3 24. P4O10 25. dinitrogen pentaoxide, dichlorine octaoxide, chlorine trifluoride 26. HNO3 27. HCl 28. Law of multiple porportions 29. molecular 30. Law of definite proportions