SJ #10 “Ionic Bonds”
advertisement
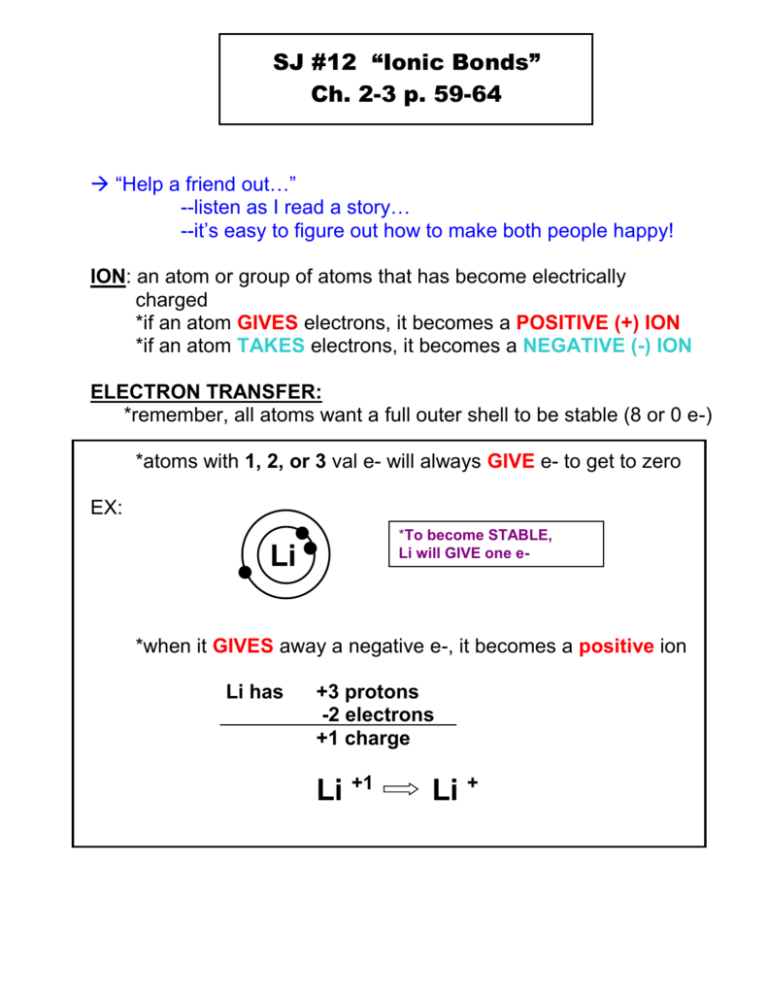
SJ #12 “Ionic Bonds” Ch. 2-3 p. 59-64 “Help a friend out…” --listen as I read a story… --it’s easy to figure out how to make both people happy! ION: an atom or group of atoms that has become electrically charged *if an atom GIVES electrons, it becomes a POSITIVE (+) ION *if an atom TAKES electrons, it becomes a NEGATIVE (-) ION ELECTRON TRANSFER: *remember, all atoms want a full outer shell to be stable (8 or 0 e-) *atoms with 1, 2, or 3 val e- will always GIVE e- to get to zero EX: *To become STABLE, Li will GIVE one e- Li *when it GIVES away a negative e-, it becomes a positive ion Li has +3 protons -2 electrons +1 charge Li +1 Li + *atoms with 5, 6, or 7 val e- will always TAKE e- to get to 8 EX: *To become STABLE, F will TAKE one e- F F has +9 protons -10 electrons -1 charge F -1 F p. 60 Ions and Their Charges IONIC BOND: the attraction between two oppositely charged IONS. *opposite ions will balance out charges so Ionic Compounds are ELECTRICALLY NEUTRAL. *ONLY happens between a metal and a non-metal! p. 61 “Exploring Ionic Bonds” ionic bond Li F + - Li F + table -add ionic charges to periodic lithium fluoride POLYATOMIC IONS: what words do you know that start with “poly-“? “poly” = many (more than 1) “atomic” = atoms * group of atoms that acts like one atom Examples: (pick any 2 to write down) (CO3)2(OH)(NH4)+ (NO3)- carbonate hydroxide ammonium nitrate NAMING IONIC COMPOUNDS 1. (+) ion, metal, always listed first 2. (-) ion, non-metal, always listed last 3. the name is ALWAYS 2 words 4. if the (-) ion is a single element (not polyatomic) the ending of the compound changes to “-ide” O2- MgO = magnesium oxide CaCl2 = calcium chloride 5. polyatomic ion names do not change EX: Mg2+ PROPERTIES OF IONIC COMPOUNDS 1. crystal shape ex: halite (table salt) *(+) and (-) ions form 3D crystal (p. 63) -each ion is attracted to opposite charge 2. high melting point *due to the strong attractions between ions ex: salt M.P. = 801° C (1400°F) *all SOLIDS at room temp 3. conduct electricity, but only when in solution! *when dissolved, the ions let electrical charges move through them. *ex: salt water in a battery











