Spectrophic/Colorimetric analysis
advertisement
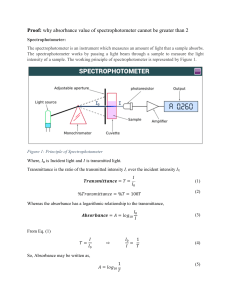
Spectroscopic /Colorimetric Analysis Emily Scheerer AP Chemistry Pd. 2 Concept Colorimetric/Spectroscopic Analysis – variation of intensity of color of a solution with changes in concentration. How a Spectrometer works: There are five parts: a light source, a monochromator, the cell, a detector and a meter The monochromator selects a wavelength from the light source to send to the cell. Light shots through the cell and the detector measures the intensity of light from the cell. The meter indicates the intensity in %T (percent transmittance) or A (absorbance) Concept cont’d There are 2 ways to express absorbance: – – – Percent transmittance T= I/I0*100 Absorbance A = log I0/I A = 2 – log10%T Beer’s Law A = abc A – absorbance a – molar absorbity b – solution path length c – concentration in moles per liter Since Beer’s law makes Absorbance proportional to concentration we can use it to determine concentration. Purpose To determine the concentration of an unknown iron substance based on Spectroscopic Analysis. Determine this by comparing the unknown measurement to the scale you create with samples of known concentration. For a given substance, the amount of light absorbed depends on: ~ Concentration ~ cell/path length ~ wavelength of light ~ solvent Spectrophotometer diagram Picture Credit: http://www.boomer.org/c/p3/c03/Fig04.gif Spectrophotometer (inside diagram) Picture credit: http://www.wellesley.edu/Chemistry/Chem105manual/Lab04/lab04_spectrophotometer.gif These are cuvettes Materials Standard iron solution Fe(NO3)3 + HNO3 (1 mL = 0.05 mg Fe) 1M NH4C2H3O2 10% hydroxylamine hydrochloride Cuvettes 0.30% o – phenanthroline 6M 50 mL volumetric flask 1,2, and 5 mL pipets Spectrophotometer 125 mL Erlenmeyer flask H2SO4 Unknown iron sample Picture credit - http://www.globescientific.com/product-type-spectrophotometer-cuvettes-c-21_630_89.html Procedure ~ Weigh out 0.1g iron unknown into a 50-mL volumetric flask ~ add 5 drops of 6M sulfuric acid and dilute to 50 mL ~ mix and transfer to 125 mL Erlenmeyer flask ~ pipet exactly 1 mL of this solution into a 50-mL volumetric flask ~ add 1 mL of 1M ammonium acetate & 1mL of 10% hydroxylamine hydrochloride & 10 mL of 0.30% o-phenanthroline solution ~ dilute to 50 mL ~ mix to develop orange red iron color (45 min) ~ Fill a clean and dry cuvette with colored solution ~ put cuvette in spectrophotometer and determine absorbance at 510 nm ~ Repeat with 1mL, 2mL, 3mL, 4mL, 5mL standard iron solution Potential Data (to explain calculations, DO NOT use this as a factual data table) Wavelength (nm) %T of standard solution 540 36.4 560 15.5 580 7.5 600 32.6 620 8.1 640 16.3 Unknown: 22.4 %T at 600 nm Potential Data (cont’d) Mass of standard solution used = 7 g Mass of unknown = 5.5 g Concentration of standard Fe = 0.0228 M Calculations Calculate the absorbance of the standard and unknown solutions from their measured transmittance. Remember: A = 2 – log10%T A (standard) = 2.00 – log(32.6) = 0.487 A (unknown) = 2.00 – log(22.4) = 0.650 Then calculate concentration of unknown given that: As/ Cs = ab = Au/Cu As = absorbance of standard solution Cs = Concentration of standard Au = absorbance of unknown Cu = Concentration of unknown Rearrange to find the concentration of the unknown: Cu = Aux Cs x 1/As= .650 x .0228 x 1/0.487 = 0.0304 M Alternate Websites Beer’s Law explanation: http://teaching.shu.ac.uk/hwb/chemistry/tutorials/molspec/beers1.htm Alternate Lab – Crystal Violet solution http://www.smes.org/classes/chemistry/ap/apCrystalVioletRateLab.htm Book Citation: Nelson, John H. Laboratory experiments for Brown and LeMay Prentice-Hall Inc, New Jersey, 1977