Unit rates
advertisement

SCH 4UK
Reaction Rates
For the Bolztmann curve, see
http://www.chm.davidson.edu/ChemistryApplets/KineticMolecularTheory/Maxwell.html
Reaction kinetics refers to the study of reaction mechanisms (i.e. how chemical reactions occur) and the
factors that affect the rates of reactions.
RATE OF REACTION - The rate of reaction refers to the rate (or speed) at which a reactant is used up or a
product is formed. Rates of reaction are typically expressed in units such as g/s, mL/s, kg/h, mol/(L.s),
L.min-1, etc. and may be negative or positive depending on whether a substance is being used up or
produced. The average rate of reaction expresses the change in a variable over a given time interval and may
be calculated using the following general formula:
V
Rav
where V = V f - Vi ..... the change in the variable
t
and t = tf - ti the time interval over which the change occurred.
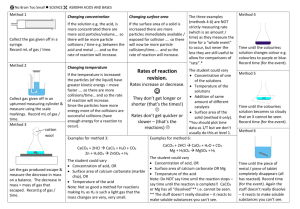
Sample Problem: Calculate the average rate of reaction for the reaction of zinc metal with hydrochloric acid
as represented by the equation, given the situations below:
Zn(s) +2 H+(aq) H2(g) + Zn2+(aq)
A)
B)
C)
2.00 L of hydrogen gas was produced in 7.0 min.
Initially [Zn2+(aq)] (the concentration ofZn2+(aq) ) is zero. After 45 s it is found to be 0.10 mol/L.
The initial mass of zinc is 3.73 g. It is measured again 5.00 min later and found to be 3.07 g
Solution to sample Problem
A)
Rv
V ( H 2 ) 2.00 L
0.2857 L / min 0.19 L / min
t
7.0 min
hydrogen gas is being produced at a rate of 0.19 L/min
B)
2
2
[ Zn 2 ] [ Zn ] f [ Zn ]i 0.10 mol / L 0.00 mol / L
Rav
0.0022 mol L.s
t
45s
45s
m( Zn) m f mi 3.07 g 3.73g 0.66 g
0.132 g / min 0.13min
t
t
5.00 min
5.00 min
For determining rates of reaction from graphs, see page 362 of text. And average rate is the slope of a
secant of a conc. time curve. And instantaneous rate is the slope of the tangent to a conc. time curve.
C)
Rav
Determining the rate of a reaction can measure the change of pressure of reaction vessel if the reaction is
producing or consuming gas. It can measure specific ion concentration like pH , if that is something that is
changing. Some reactions produce or consume coloured ions that can be monitored.
Collision Theory Collision Theory is a model to explain how chemical reactions occur.
The essential features of the collision theory of chemical reactions are:
1. In order for a chemical reaction to take place the particles of the reactants must collide with each
other.
2. Most collisions among molecules do not result in chemical reaction, i.e. are not effective.
3. In order for collisions to be effective the colliding molecules must have sufficient energy to break
SCH 4UK
Rates of Reactions
existing chemical bonds. The minimum energy necessary to break existing bonds and initiate a
reaction is called the activation energy, Ea.
4.
In order for collisions to be effective the geometry of the collision (i.e. the positions of the molecules
relative to each other) must ideal. Otherwise the collision may not result in reaction. Most chemical
reactions are thought to occur by a sequence of collisions involving only two particles (occasionally
3 particles; rarely four or more! ! ) The probability of four or more particles colliding simultaneously
is extremely remote.
5.
The sequence of steps by which a chemical reaction occurs is called a reaction mechanism and may
be written as a series of balanced equations.
RATE-DETERMINING STEP - This is the slowest step in the mechanism since it controls the rate at which
the overall reaction can take place. In the above mechanism the rate-determining step is step 1. Ratedetermining steps cannot, as a rule, be predicted from the reaction mechanism. However, one factor that is
involved in the rate-determining step is the strength of the bond(s) that must be broken.
Do demo of the creation of CHCl3
Step one: put a bond on the H and pass
Step two: put a C on the H with the bond
Step three : put 3 more bonds on the C and attach H’s to the bonds
Step four: replace one H with a Cl
F ACTORS AFFECTING RATES OF REACTION
The rate at which a chemical reaction occurs is determined by a number of different factors which we will
consider here. These factors include: 1) concentration, 2) temperature, 3) surface area, 4) catalysts, and 5) the
nature of reactants. See Video # 56. There is a Hoffman segment after the elements on some factors
affecting rate with emphasis on catalysts.
CONCENTRATION
The rate of reaction is dependent on the frequency with which the reacting particles collide (i.e. # collisions
per unit of time). While not all collisions are effective, it follows that the more frequently particles collide,
the more frequently effective collisions occur, and therefore the greater the rate of reaction. Keeping in mind
that the overall rate of a reaction is determined solely by the rate-determining step, if we change the
concentration of a component in the rate-determining step then the rate of reaction will change accordingly.
(Changing concentrations of other components that are not in the rate-determining step should not affect the
rate of reaction.) This generalization takes the form of the LAW of MASS ACTION (aka RATE LAW)
which states that the rate of a chemical reaction is directly proportional to the concentration (to the power
"m", "n"... etc.) of each reactant in the rate determining-step, where m, n,... etc. represent the balancing
coefficient(s) from the rate-determining step.
If rate-determining step is: m A + n B p C (m, n, p are balancing coefficients)
n
Then the Rate of reaction is R k[ A]m [ B] [ ] = "concentration of"
That is, the rate is directly proportional to the concentration of reactant A to the power "m" and to the
concentration of reactant B to the power "n". " k" is a "rate" or proportionality constant. The values of m and
n are usually deduced from experimental data, as is the value of the rate constant, k.
Example: Write the Rate Law formula for the reaction of hydrogen bromide with oxygen given the overall
equation and reaction mechanism below.
2
SCH 4UK
Rates of Reactions
Overall reaction:
4 HBr(g) + O2(g) 2 H2O(g) + 2 Br2(g)
Reaction mechanism: elementary step 1:
HBr +
O2 HOOBr slow
elementary step 2:
HOOBr
+
HBr 2 HOBr fast
elementary step 3:
HOBr
+
HBrH2O + Br2 fast
HOBr
+ HBr H2O + Br2 fast
Solution: The rate-determining step is step 1: (l)HBr + (1)O2 HOOBr slow
Therefore the Rate Law formula is R = k[HBr11[O2]1 or R = k[HBr][O2]
Exercise: For each of the hypothetical reaction mechanisms given below;
(i) identify the rate-determining step:
(ii) determine the overall equation.
(iii) write the rate law.
1)
X + Y 2D+G
fast
Ans. R= k[D]2 but as [2D] depends on X and Y, then
2DE + F
slow
R= k[ X][Y]
G+ FZ
fast
2)
B2 + C D + 2 E
E + C F + H
E + HK
slow
fast
fast
3_
A2 + B X2 + Y
X2 2X
2X + Z2 2G
fast
fast
slow
4) Experimental evidence suggests that the reaction:
2 NO2Cl 2 NO2 + Cl2 occurs according to the following mechanism:
NO2Cl NO2 + Cl
(slow)
NO2Cl + CI NO2 + Cl2
(fast)
Write the rate law expression (formula) for this reaction.
See text pg 389. Exercise pg 391: 1,2
TEMPERATURE
According to the Kinetic Molecular Theory of Matter, temperature is a direct measure of the average
kinetic energy of a substance: at low temperatures molecules have relatively low KE, at high temperatures
molecules have higher KE. Collision theory states that in order for a collision of molecules to be "effective"
or "successful" (i.e. break existing bonds and result in chemical reaction) the colliding particles must have a
certain minimum energy called the ACTIVATION ENERGY (Ea). This may be represented in the form of
a graph known as a "Boltzmann Distribution Curve" (or Boltzmann Curve). The Maxwell-Boltzman
distribution curve is a plot of the fraction of particles with a particular kinetic energy against kinetic energy
values. The Boltzmann Curve for molecular collisions plots the kinetic energy of collisions versus the
fraction (or percentage) of collisions. An analogy to help understand the concept of Boltzmann Curves may
be to consider traffic on a major highway (e.g. the 401). Suppose you measured the speed of cars traveling
on the highway. If a graph of speed versus number of cars is plotted the graph would take on a bell shape
(very few cars would be traveling at speeds less than 80 km/h, most would be traveling at speeds between
80-130 km/h, and very few would travel faster. Hence the curve is tapered at both ends with a big bulge in
the centre. Although the analogy is somewhat crude, when the collision kinetic energy of molecules is
plotted against the fraction of collisions with various energies, a curve of similar shape is obtained.
3
SCH 4UK
Rates of Reactions
Boltzmann Curve for Traffic on a Major Highway
Fraction of
cars
0
80
Speed (km/h)
120
Show also the overhead of the Boltzman distribution of light energy from the sun.
Now look at a graph of a Boltzmann Curve for Molecular Collisions.
Boltzmann Curve for Molecular Collisions
Fraction of
Collisions
Ea
Region B
region of
ineffective
collisions
Region of effective collisions
B
0
0
Kinetic Energy of Collisions
Region B represents the vast majority of collisions which are not effective. collisions in the region have KE
less than Ea , the minimum energy necessary for reaction to occur.
Region A represents the proportion of all collisions that do have KE equal to or greater than Ea, the minimum
energy necessary for collisions to be successful (result in chemical reaction).
The situation depicted by this graph suggests that approximately 10% of all collisions are effective which the
remaining 90% are ineffective (unsuccessful)
EFFECT OF TEMPERATURE - Since temperature is a measure of kinetic energy it follows that a change
in the temperature of a substance means a change in the kinetic energy of its molecules. Increasing
temperature increases the average energy of molecular collisions. This leads to the following generalizations:
TEMPERATURE INCREASE -The higher the temperature of the reactants, the greater the average kinetic
energy of its molecules, the greater the proportion of successful collisions, the greater the rate of reaction.
TEMPERATURE DECREASE - The lower the temperature of the reactants, the lower the average kinetic
energy of its molecules, the smaller the proportion of successful collisions, the lower the rate of reaction.
Referring back to our "cars on the highway" analogy, the effect of changing the temperature of a mixture of
reacting molecules is very similar to the effect of changing the speed limit on Hwy #401. When the speed
4
SCH 4UK
Rates of Reactions
limit is raised the average speed increases so that the bulge on the Boltzmann curve shifts to the right. When
the temperature of the reactants is increased the Boltzmann Bulge shifts to the right as well. The effect of this
is that a relatively small increase in temperature can cause a large increase in the proportion of effective
collisions (large increase in region B - see the graph) and therefore a large increase in the rate of reaction.
However, just as the total number of cars on the road does not increase significantly (if at all) when the speed
limit is raised, the total number of molecular collisions does not increase significantly. Study the diagram
below. Pictures in text on pg 383
EFFECT OF TEMPERATURE INCREASE
In the diagram above the three curves represent the same system at a cold temp, room temp. and hot.. The
total area under each curve is about the same indicating that the total number of collisions does not change
significantly for small temperature changes. However, the area representing effective collisions has increased
dramatically from cold to warm to hot.
GENERALIZATION - For many chemical reactions (but not all) a temperature change of only 10°C or 10 K
(e.g. from 35°C to 45°C; from 273 K to 283K, etc) will cause the proportion of effective collisions to double
( approximately ), thus doubling the rate of reaction.
Fraction
of
Collisions
Fraction
of
Collisions
Ea
Collision KE
Collision KE
POTENTIAL ENERGY (PE) DIAGRAMS
PE Diagram for a Non-spontaneous Exothermic Reaction
II
Eaf
Reactants
Ear
Hrn
PE
5
SCH 4UK
Rates of Reactions
POTENTIAL ENERGY (PE) DIAGRAMS
The energy changes that occur during the collision of reactant particles can be "mapped" out in the form of a
potential energy diagram such as the one below. Since many chemical reactions are reversible the horizontal
axis is labeled "progress of reaction" rather than time. NOTE: We will adopt the practice common to most
chemistry texts of using PE Diagrams to represent the overall reaction, although each step in a reaction
mechanism involves its own unique energy transformations and can be described in terms of its own PE
diagram.
Region I represents the situation when the reactant molecules are on a collision path but far apart so that no
repulsions or attractions are occurring between them, hence the molecules have constant potential energy
(straight section of PE curve).
Region II - As the molecules begin to get closer together electron-electron repulsions begin to increase
causing potential energy to increase (kinetic energy is being converted into potential energy and the
molecules begin to slow down). The PE continues to build and molecules lose speed as repulsions continue
to increase. The chemical bonds in the colliding molecules are being stretched. The top of the curve
represents the point at which the existing bonds have been stretched to the breaking point and new bonds are
about to form. The species that exists at that instant, with partly broken and partly formed bonds, is called the
activated complex (AC). It represents the state of maximum potential energy during the entire reaction. From
that point on the reaction proceeds spontaneously as chemical bonds form and potential energy is converted
back into kinetic energy.
Region III- As the new product molecules move far apart the potential energy once again level off as they no
longer interact with each other.
ACTIVATION ENERGY (Ea) - Activation energy is the minimum kinetic energy which reacting particles
must possess in order to break existing bonds thereby initiating a reaction. Colliding molecules that do not
possess this minimum energy will merely bounce off each other and go their separate ways. Activation
energy is always positive (it always takes energy to break bonds).
Eaf = forward activation energy; i.e. activation energy for the forward reaction.
Eaf = PE(activated complex) - PE(reactants)
Ear = reverse activation energy; i.e. activation energy for the reverse reaction.
Ear = PE(activated complex) - PE(products)
H= PE(products) - PE(reactants)
6
SCH 4UK
Rates of Reactions
Spontaneous Forward Reaction
Eaf (small)
PE
(-ve)
Ear(large)
Progress of Reaction
SPONTANEOUS REACTIONS - Reactions in
which most molecular collisions are successful are said to be spontaneous. In such reactions the activation
energy or Ea is very low and may be represented by a low "hump" or energy barrier on the PE curve. Such
reactions include precipitation reactions in which the precipitate appears to form almost instantaneously. A
low Eaf means the reaction is spontaneous in the forward (left to right) direction whereas a low Ear means the
reverse reaction is spontaneous.
THE EFFECT OF CATALYSTS AND INHIBITORS ON RATES
CATALYSTS are substances which can increase the rates of reaction without themselves being used up in
the process.
A catalyst works by changing the reaction mechanism in such a way that it lowers the activation energy. The
catalyst may help to form a different activated complex with lower activation energy than before, or may
even be part of the new activated complex. Lower activation energy means that a higher proportion of the
collisions will be effective even though the total number of collisions and the average collision energy
remain unchanged. The same overall products are formed.
INHIBITORS are substances that slow down chemical reactions without being used up. Inhibitors change
the reaction mechanism so that the new activated complex has a higher activation energy than before. A
higher activation energy means a smaller proportion of successful collisions and a lower rate of reaction. The
total number of collisions (total area under the curve) and temperature remain the same. NOTE: Catalysts
and inhibitors have no effect on the heat of reaction, H, since the overall products are the same and
therefore the potential energy of reactants and products remains unchanged. This is consistent with Hess'
Law which, put another way, states that the heat of reaction is independent of the path taken to get from
reactants to products.
7
SCH 4UK
Rates of Reactions
Inhibited Rea tion
Uncatalysed Reaction
Catalysed Reaction
PE
Eaf(inhib)
Eaf
Eaf(catalyst)
Progress of Reaction
BOLTZMANN CURVE OF EFFECT OF CATALYSTS & INHIBITORS
The total number of collisions for a given reaction remains unchanged provided other variables ( e.g.
temperature, concentration) remain constant. Therefore the shape of the Boltzmann curve is not affected by
the presence of a catalyst or inhibitor. Remember that the area to the right of the activation energy barrier
(Ea) represents the percentage of effective collisions.
Region A = percent effective collisions for the "inhibited" reaction. This diagram illustrates that
approximately 5% of the collisions in this reaction are effective for the inhibited reaction (Region A
represents ~5% of the total area under the Boltzmann curve.
Region A+B = percent effective collisions for the normal or "uncatalysed" reaction. About 15% of the total
number of collisions are effective during this uncatalysed reaction ( region A +B= 15% ).
Region A+B+C = percent effective collisions for the catalysed reaction ( ~40%).
Boltzmann Curve of Effect of
Catalysts and Inhibitors
Inhibited Reaction
Catalysed Reaction
Uncatalysed reaction
Number of
Collisions
Eaf(catalysed)
Eaf(uncatalysed)
Eaf(inhibitor)
C
B
A
KE of Collision
8
SCH 4UK
Rates of Reactions
kj
APPLICATIONS of CATALYSTS and
INHIBITORS
50
With inhibitor
Catalysts, in the form of metals or metal oxides, have
found extensive application in the chemical industry ,
40
from fertilizers to polymer chemistry. Catalytic
With noinhibitor
converters in automobiles use platinum or palladium
30
metal catalysts imbedded in ceramic beads to convert
carbon monoxide and unburned hydrocarbons to carbon
20
dioxide and water. Newer catalysts are capable of
converting nitric oxide (one of the components of acid
10
rain) into nitrogen gas and oxygen gas. Biological
catalysts, or enzymes, are protein molecules that control
almost all reactions in living cells. The synthesis of
ammonia, one of the most important industrial chemicals in the world, from nitrogen gas and hydrogen gas,
is catalysed by a catalyst consisting of pure iron "doped" with aluminum oxide ("alumina") and potassium
carbonate. NH3 is used both directly and indirectly (in the form of NH4+ salts such as (NH4)3PO4) as a
fertilizer. It was first synthesised commercially by Germany in 1913, just in time to replace natural supplies
of NO3- from Chile cut off by the Allied naval blockade during WW I. Ammonia is used to make nitric acid
which in turn is used to manufacture explosives, gunpowder, etc. Annual production of ammonia in the U.S.
in the 1980's reached nearly 20 million metric tonnes (in excess of 1012 mol), second only to sulfuric acid.
Inhibitors are used to slow the rate of decomposition in foods and medicines. For example, the nitrite ion,
NO2-, is added to packaged meats to "increase" shelf life, whereas SO32- slows the wine vinegar process.
SURFACE AREA
This factor applies only to HETEROGENEOUS REACTIONS, reactions that take place only at the
interface between two different phases(or states) of matter. The most familiar of these are the reactions of
metals with acids (solids and liquids), although reactions of gases with solids or gases with liquids are not
uncommon. When identifying heterogeneous reactions look for the subscripts (aq) and (s), (l) and (s), (s) and
(g), etc. on the same side of the equation.
In heterogeneous reactions the rate of reaction is directly proportional to surface area. (Do the flour
demo. It may help to dry the flour in the drying oven and to crush it further with a mortar and pestle.)
In terms of the collision theory of reactions, increasing the surface area of a reactant will increase the total
number of collisions, therefore increasing the number of effective collisions which, in turn, increases the rate
of reaction. The proportion (percent) of effective collisions does not change (as long as T remains constant).
The effect of changing surface area is illustrated schematically below for the heterogeneous reaction of zinc
metal with hydrochloric acid according to the equation
Zn(s) +2 H+ (aq) H2(g) + Zn2+(aq)
o
o
o
o
o
o
o
o
o
o
o
o
o
o
o
o
o
o
o
Homogeneous reactions that are catalysed by a catalyst in a different phase are also
directly affected by
Most surface are exposed
More surface area
Least surface area exposed
thus rate is greatest.
exposed, thus rate
to acid, thus rate is slowest
is intermediate
9
SCH 4UK
Rates of Reactions
surface area. For example, the reaction of hydrogen gas with oxygen gas, virtually non existent at room
temperature (remember you had to use a burning splint to make this reaction "happen"), is catalysed by
platinum metal.
( Platinum )
H 2 ( g ) O2 ( g )
H 2O(l )
As hydrogen and oxygen molecules collide with the platinum surface their bonds are "destabilized", allowing
the formation of an activated complex. Therefore increasing the surface area of the catalyst should increase
the rate of reaction in direct proportion.
Another reaction that is catalysed by platinum metal is the decomposition of hydrogen peroxide.
This compound is fairly stable at room temperature, but reacts very quickly in the presence of Pt metal. It is
also catalysed by a number of organic compounds including a liver enzyme and yeast. (also MnO2)
SURFACE AREA and EXPLOSIONS - An application: One of the most dramatic applications of surface
area as a factor affecting the rate of reaction is in dust explosions such as those that occur in grain elevators.
The combustible dust prevalent in elevators creates a huge surface area for colliding oxygen molecules. A
spark is required to initiate the reaction, allowing a few successful, exothermic collisions to occur. These
initial few collisions each, in turn, provide sufficient energy for several more successful collisions to occur.
The process rapidly multiplies into an uncontrolled, explosive reaction.
NATURE OF REACTANTS
When reactants with different chemical composition are subjected to otherwise identical conditions they will
react at different rates. The difference in rates must be the result of differences in very NATURE OF
REACTANTS, i.e. differences in the strength and number of bonds, if any, that must be broken in order for
the reaction to proceed. The stronger the bonds in the reactant molecules, the more energy will be required to
break them, the smaller the proportion of successful collisions, the slower the reaction rate. Consider the
following example in which 0.35 mol/L solutions of ferrous ion (Fe2+) and oxalate ion (C2O42-) are both
subjected to 1.0 mol/L H+(aq) and 1.0 mol/L MnO4-(aq) under identical conditions of temperature and
pressure
Rx 1: 5 Fe2+(aq)
+
(0.35 mol/L)
8 H+(aq) +
(1.0 mol/L)
MnO4-(aq) 5Fe3+ (aq) + Mn2+(aq) + 4H2O(l) ...fast
(1.0 mol/L)
Rx 2: 2½ C2O42-(aq) + 8 H+ (aq) + MnO4-(aq) 5 CO2(g) + Mn2+{aq) + 4 H2O(l) ... slow
(0.35 mol/L) (1.0 mol/L) (1.0 mol/L)
Since all conditions are identical in both reactions the difference in rate must be due to the difference in the
chemical nature (composition) of Fe2+ and C2O42-. Closer inspection of reaction 1 reveals that each Fe2+ ion
is simply losing an electron (to an oxalate ion) in order to become Fe3+. Loss of electrons from metal ions,
compared to bond breaking, requires relatively little energy and is therefore relatively easy.
Rx 1: Fe2+ Fe3+ + eCareful consideration of reaction 2 shows that each C2O42- ion is being broken apart into CO2 molecules.
This involves breaking the strong C-C bond (347 kJ/mol), a relatively difficult task. Therefore a smaller
proportion of collisions involving these ions will be successful. See the structural diagram below.
O
C
O
O
O
O
O C O
C
O
C
10
SCH 4UK
Rates of Reactions
Exercise: Consider the three reactions below. Explain in terms of bond breaking and electron transfer why
the rates of these reactions are different.
(i)
2NO + O2 2NO2
Intermediate rate at 20C and std pressure
(ii)
CH4 + 2O2 CO2
+ 2 H2O
Extremely slow at 20C and std pressure
4+
2+
3+
3+
(iii)
Ce
Fe Ce
+ Fe
Fast at 20 C and std pressure
(i) is intermediate as the product is formed from the decomposition of a 3 molecule intermediate.
(ii) is slow as there are many bonds to break, and the C-H bond is strong as is the O=O bond
(iii) is fast as it is an electron exchange reaction
Relating Reaction Rate to Time:
Have students in small groups come up with graphs of initial conc. versus time for zero, first and second
order reactions.
Zero order reactions have horizontal graphs
First order reactions have straight line positive slopes.
Second order and higher graphs are curves e.g. positive half of quadratics. they can be reduced to straight
line graphs by plotting initial conc. raised to correct power (order) versus time. See text pg 378.
Exercise pg 381 1-3
The order of the reaction tells us the number of particles in the slow step of the reaction and gives us a better
guess as to how the reaction is taking place. This in turn gives us a better understanding of how chemical
reactions take place and how to better control them.
11