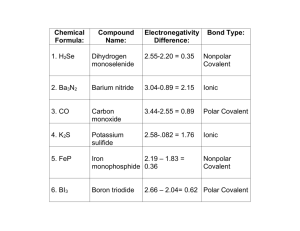
Topic 6 Practice - Bonding A) 1 B) 2 C) 3 D) 4 1. Given the
advertisement

Topic 6 Practice - Bonding 1. Given the formula for hydrazine: 10. Which type of substance is soft, has a low melting point, and is a poor conductor of heat and electricity? A) network solid C) metallic solid How many pairs of electrons are shared between the two nitrogen atoms? A) 1 B) 2 C) 3 D) 4 2. Which bond is least polar? A) As–Cl B) Bi–Cl C) P–Cl D) N–Cl 3. Given the electron dot diagram: The electrons in the bond between hydrogen and fluorine are more strongly attracted to the atom of A) B) C) D) hydrogen, which has the higher electronegativity fluorine, which has the higher electronegativity hydrogen, which has the lower electronegativity fluorine, which has the lower electronegativity 4. Which substance contains bonds that involved the transfer of electrons from one atom to another? A) CO 2 B) NH 3 C) KBr D) Cl 2 5. Compared to a calcium atom, the calcium ion Ca 2+ has A) more protons C) more electrons B) fewer protons D) fewer electrons 6. Which compound contains ionic bonds? A) NO B) NO 2 C) CaO D) CO 2 7. A hard substance that has a high melting point and is a poor conductor of electricity in the solid phase could be A) CO 2 B) Mg C) NaCl D) CCl4 8. Which compound has the strongest hydrogen bonding between its molecules? A) HBr B) HCl C) HF D) HI 9. Which two substances are covalent compounds? A) B) C) D) C6 H12 O6 (s) and KI(s) C6 H 12 O 6 (s) and HCl(g) KI(s) and NaCl(s) NaCl(s) and HCl(g) B) molecular solid D) ionic solid 11. Which type of bond is found between atoms of solid cobalt? A) nonpolar covalent C) metallic B) polar covalent D) ionic 12. The high electrical conductivity of metals is primarily due to A) B) C) D) high ionization energies filled energy levels mobile electrons high electronegativities 13. Silicon dioxide (SiO 2) and diamonds are best described as A) molecular substances with coordinate covalent bonding B) molecular substances with ionic bonding C) network solids with covalent bonding D) network solids with ionic bonding 14. Which molecule has a nonpolar covalent bond? A) B) C) D) 15. The degree of polarity of a chemical bond in a molecule of a compound can be predicted by determining the difference in the A) melting points of the elements in the compound B) densities of the elements in the compound C) electronegativities of the bonded atoms in a molecule of the compound D) atomic masses of the bonded atoms in a molecule of the compound 16. Which type of molecule is CF 4? A) polar, with a symmetrical distribution of charge B) polar, with an asymmetrical distribution of charge C) nonpolar, with a symmetrical distribution of charge D) nonpolar, with an asymmetrical distribution of charge 17. What is the empirical formula for a compound with the molecular formula C 6H12Cl 2O2? A) CHClO C) C3H 6ClO B) CH 2ClO D) C6H12C12O2 18. The compounds C 2H4 and C4H8 have the same A) B) C) D) freezing point at standard pressure boiling point at standard pressure molecular formula empirical formula 19. What is the chemical formula for zinc carbonate? A) ZnCO3 C) Zn2CO 3 B) Zn(CO 3) 2 D) Zn3CO 2 20. The formula H 2O2 is an example of A) B) C) D) a molecular formula an empirical formula an ionic formula an organic formula 21. A 1.0-mole sample of krypton gas has a mass of A) 19 g B) 36 g C) 39 g D) 84 g 22. What is the gram-formula mass of Ca3(PO4) 2 ? A) 248 g/mol C) 279 g/mol B) 263 g/mol D) 310. g/mol 23. A compound has a molar mass of 90. grams per mole and the empirical formula CH2O. What is the molecular formula of this compound? A) CH 2O C) C3H 6O 3 B) C2H4O2 D) C4H8O4 24. What is the percent composition by mass of sulfur in the compound MgSO4 (gram-formula mass = 120. grams per mole)? A) 20% B) 27% C) 46% D) 53% 25. The correct chemical formula for iron(II) sulfide is A) FeS C) FeSO 4 B) Fe 2S3 D) Fe 2(SO4) 3 26. What is the IUPAC name for the compound ZnO? A) zinc oxide C) zinc peroxide B) zinc oxalate D) zinc hydroxide Answer Key Topic 6 Practice - Bonding SHORT 1. A 2. D 3. B 4. C 5. D 6. C 7. C 8. C 9. B 10. B 11. C 12. C 13. C 14. A 15. C 16. C 17. C 18. D 19. A 20. A 21. D 22. D 23. C 24. B 25. A 26. A