Chapter 7 and 8 Naming Review Worksheet
advertisement
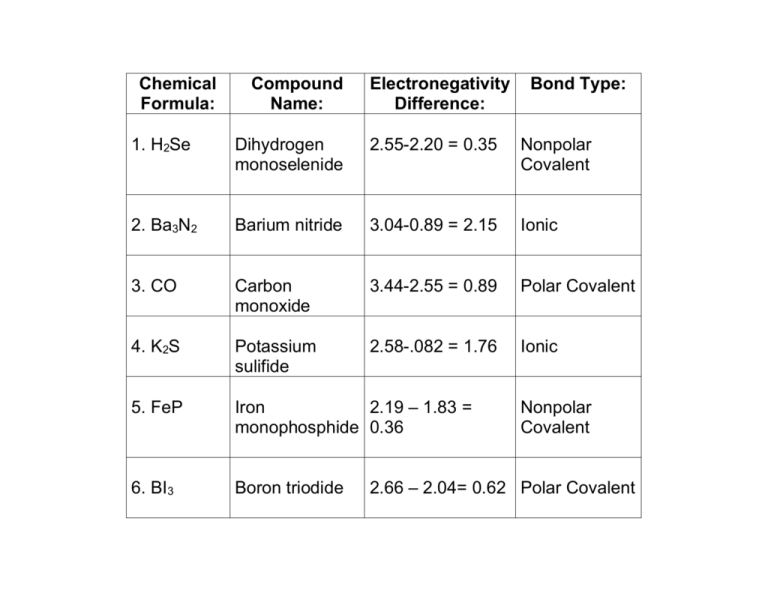
Chemical Formula: Compound Name: Electronegativity Difference: Bond Type: 1. H2Se Dihydrogen monoselenide 2.55-2.20 = 0.35 Nonpolar Covalent 2. Ba3N2 Barium nitride 3.04-0.89 = 2.15 Ionic 3. CO Carbon monoxide 3.44-2.55 = 0.89 Polar Covalent 4. K2S Potassium sulifide 2.58-.082 = 1.76 Ionic 5. FeP Iron 2.19 – 1.83 = monophosphide 0.36 6. BI3 Boron triodide Nonpolar Covalent 2.66 – 2.04= 0.62 Polar Covalent I. Follow up questions on table: 1. Rank the six compounds and their respective bonds from the most nonpolar covalent to the most ionic: Compounds 1, 5, 6, 3, 4, and then 2 are in the right order, based on the criteria above. 2. For compounds 1, 2, and 3, briefly describe their respective bonds in terms of valence electrons (concepts to consider using—electron transfer; octet; share equally; share unequally): Compound 1—this compound has two nonpolar covalent bonds in which electrons in each are basically shared equally (electronegativity difference of less than 0.4); Selenium achieves its octet to have an electron cloud like Krypton and each Hydrogen atom achieves the noble gas configuration of Helium. Compound 2—this compound has ionic bonds due to the transfer of electrons from the two Barium atoms to the three Nitrogen atoms (electronegativity difference greater than 1.7). Each Barium atom transfers two electrons to achieve the octet of Xenon and each Nitrogen atom accepts three electrons to obtain the eight valence electron configuration of Neon. Compound 3—this compound has polar covalent bonds in which electrons are shared unequally (the electronegativity difference between the two elements is between 0.4 and 1.7). By sharing electrons, each element achieves the octet of Neon. II. “Houston…we have a problem”: Identify the problem(s) with each chemical name or formula AND make the corrections: 1. AgBr2 is Silver (II) bromine: Silver only has one oxidation number (1+; NOT 2+); Bromine as the anion should end with the suffix ‘-ide’. So…AgBr and ‘Silver bromide’. 2. P2O5 is Pentaphosphide dioxygen: Mixed prefixes for the two elements and incorrectly named them. Should be Diphosphorous pentoxide. 3. Fe3(PO4)4 is Iron (IV) phosphate: Iron has either a 2+ or a 3+ oxidation number NOT 4+. So…the compound could be Iron (II) phosphate [Fe3(PO4)2] OR Iron (III) phosphate (FePO4). 4. CO2 is Monocarbon tetraoxide: Incorrect use of prefixes. The correct compound name is Carbon dioxide.











