Name - Faculty
advertisement

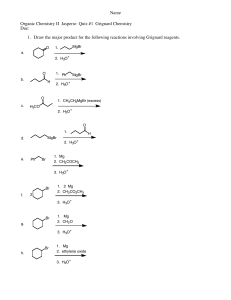
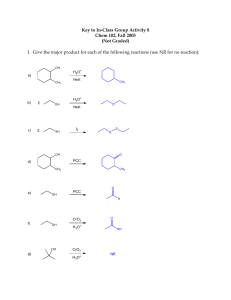
1 Name________________________________________________________________________ Last First CHEM 308.001/002 Dr. Alfred Amah Sixth Examination May 7, 2004 50 minutes 50 points 1. Draw structures(show hydrogens about all carbons, except for rings that may be shown in the usual manner) that correspond to the following names: (6 points) (a) 2, 4, 6-Trinitrophenol (b) 2-Phenyl-2-propanol (c) 3-Methyl-2-buten-1-ol 2. Acid-catalyzed dehydration of 2, 2-dimethylcyclohexanol yields 1,2-dimethylcyclohexene as one of the major products. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and show all intermediate structures for full credit. (8 points) CH3 OH CH3 H3O+ CH3 CH3 2 3. Grignard Reagents. A highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List two different possibilities for each compound. (12 points) (a) OH C CH2CH3 CH3 (b) OH CH2 C CH3 CH3 3 4. Organic Products. Give the major organic product(s) of the following reactions or sequence of reactions. Show all relevant stereochemistry.(12 points) OH (a) OH ? CrO3, H2SO4 THF O (b) MgBr 1. ether + H ? 2. H3O+ Br (c) OH (CH3)3SiCl ? pyd. CH3 (d) OH NaH ? O O (e) 1. NaBH4, ethanol OH ? 2. H3O+ O (f) 1. (CH3)3SiCl, pyd. ? 2. CH3MgBr, ether OH 3. H3O+ 4 5. Give the reactant(s), product(s) or reagent(s) as indicated by the (?) in the following synthetic scheme. Do not balance or show mechanism for each step. (12 points) (a) Br O ? 1. ? MgBr ? 2. H3O+ (b) O 1. Hg(OAc)2 H2O 2. NaBH4 ? ? 1.CH3CH2MgBr 2. H3O+ ?