Chemistry response 3 investigating orbitals
advertisement

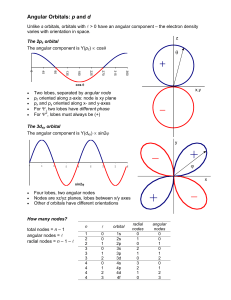
Investigating Orbitals A comment about the shapes of orbitals It is often quoted that an orbital shows a volume in which there is a certain probability, say 90%, of finding an electron. Actually, it is possible to create any shape in which there is a 90% (or any other) probability of finding the electron – it could be a sphere, a cube, a pyramid, or mickey mouse. The important point, is that the shapes are isosurfaces, that is, joined up regions in space that share a particular numerical value of the mathematical function that describes the electron. It might be possible to show the particular isosurface which also contains the chosen percentage of the total chance of finding the electron, but that is a separate issue. When you were using the modelling programme, you may have seen that the orbital appears to be larger when a smaller value of the wavefunction is chosen for the isosurface value. This is correct (although confusing) unless the previous point is appreciated. Remember that the square of the value of the wavefunction at a particular position gives the chance of finding the electron around that region. A lower value for the wavefunction therefore indicates a lower chance of finding the electron. The further away from the nucleus, the lower the chance of finding the electron – after all, the electron is in the atom, somewhere near the nucleus. Regions where the wavefunction is zero – nodes. After exploring the orbitals, some simple rules may be formulated regarding the number and type of nodes in a particular orbital. In the modelling programme, having ‘Nodal Surfaces’ selected helps you see the angular and radial nodes. Number of nodes You will have observed that the total number of nodes is equal to the principal quantum number, n, minus one. In other words, the 1s has 0 nodes the 2s and the 2p orbitals each have 1 node the 3s, 3p and 3d orbitals each have 2 nodes the 4s, 4p, 4d, and 4f orbitals each have 3 nodes and so on. What are quantum numbers? There are a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, l, m, and s specify the complete and unique quantum state of a single electron in an atom called its wavefunction or orbital. No two electrons belonging to the same atom can have the same four quantum numbers. The shell number corresponds to the principal quantum number, n. Number of angular nodes Angular nodes may either be planes, or they can be cones (see the diagrams below). What they actually correspond to are particular angles from the x, y, and z axes where the wavefunction is zero. All s orbitals, i.e. the 1s, 2s, 3s, 4s, and so on, have no angular nodes. All p orbitals, i.e. the 2p, the 3p, the 4p, and so on, have one angular node. This angular node, shown in green below, is a plane which separates the two haves of the orbital. All d orbitals have two angular nodes. For four of the d orbitals – the dxy, dxz, dyz, dx2–y2, these are two nodal planes but for the dz2 the two nodes are two cones. The two points of the cones just touch each other at the origin as shown below. All f orbitals have three angular nodes. These are combinations of nodal planes and cones, depending on the particular f orbital. This a good point to introduce another quantum number. The number of angular nodes for a given orbital is actually equal to the orbital angular momentum quantum number, given the symbol l. The number of angular nodes may be summarised as follows: orbital s p d f number of angular nodes, l 0 1 2 3 Number of radial nodes Radial nodes are spherical. The difference between the total number of nodes and the number of angular nodes is the number of radial nodes. Given the total number of nodes (angular plus radial) is equal to n – 1 (where n is the principal quantum number) and the number of angular nodes is equal to l, it follows that the number of radial nodes for a particular orbital is n – 1 – l. The radial node for the 4p orbital is shown by the green circle in the figure below. The table overleaf summarises the total number of nodes and the numbers of angular and radial nodes for all the orbitals with principal quantum number n = 1 to 4. orbital principal quantum number total number of nodes number of angular nodes number of radial nodes n =n–1 =l =n–1–l 1s 1 0 0 0 2s 2 1 0 1 2p 2 1 1 0 3s 3 2 0 2 3p 3 2 1 1 3d 3 2 2 0 4s 4 3 0 3 4p 4 3 1 2 4d 4 3 2 1 4f 4 3 3 0 Going further Once the pattern between the number and type of nodes in an orbital is understood, it should be possible to appreciate the form of any orbital. Websites such as http://winter.group.shef.ac.uk/orbitron/ can be used to visualize orbitals up to the shell n = 7.