The Hydrogen Atom - Valdosta State University
advertisement

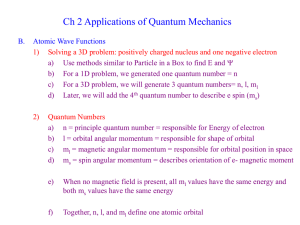
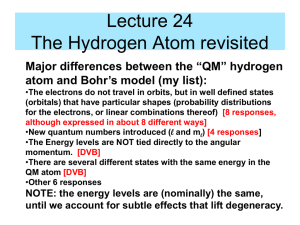
9-1 Chapter 9 The Hydrogen Atom Goal - to solve for all eigenstates (orbitals) H atom - single nucleus, charge Z (+1) and one eattracted by Coulomb’s Law Will find third quantum number, n, that is ≥ 1 1. Write the full Hamiltonian. Now need to let r vary since real atoms don’t have fixed distances between nuclei and electrons. 2. Use separation of variables to pull out part already solved for rigid rotor (angular part). 3. Show solution for radial part (r dependent part). E energy will depend only on the n quantum number (and not m or l). Finally, we draw pictures of the orbitals and count up the orbital nodes and level degeneracy. Hamiltonian is the sum of the kinetic energy of each particle plus the potential energy, which here is Coulombic attraction. (Recall Coulomb’s Law) 0 = permittivity constant Distance between charges 9-2 The Schrodinger equation in polar coordinates: 1 2 (r , , ) 1 (r , , ) r sin 2 2 2 r r r e2 r sin (r , , ) 2 2me 1 4 r (r , , ) 0 2 2 r sin E (r , , ) Radial Equation: Separation of Variables Notice the potential only depends upon r. That means it’s separate from the and parts. The total wave function then can be written as a product of the part we did for angular momentum and the radial part: This allows us to get the radial only equation. (r , , ) R(r )( )( ) We already did the and parts to generate the spherical harmonics. 9-3 We can make substitution recalling that lˆ( )( ) 2l (l 1)( ) ( ) To find: 2 d 2 dR(r ) 2 l (l 1) e2 r R(r ) ER(r ) 2 2 dr 2me r 40 r 2me r dr Second term can be viewed as an effective potential. (Centripetal plus Coulombic parts) 9-4 Recall, for the harmonic oscillator, the fundamental solution was a Hermite polynomial, here now the fundamental part is an exponential. See page164. Z is the charge on the nucleus. 0h2 a0 me e 2 This is the Bohr radius. An,l is a constant Total energy eigenvalues are negative by convention. Zero is taken at r ∞. Negative energies correspond to bound states. 9-5 Potential is drawn as before. Yellow is classically forbidden region. Energy eigenvalues are superimposed. Notice that they get closer as n ∞. Need 3 Quantum numbers to describe state of H 9-6 Complete normalized total energy eigenfunctions include spherical harmonics. Note that EF real only if ml = 0! Convenient to linearly combine orbital functions with their complex conjugate to create real functions. 9-7 Absorption spectrum of Hydrogen 1s orbital 9-8 “Leading edge” of contour plot for s functions is R(r) Note nodes in R(r) 9-9 To display H total energy EF other than ns, need contour plot of .(x,y,z)with x or y or z = 0. Note different appearance of angular and radial nodes. Clockwise from top left: 2py, 3py, 3dz , 3dxy 2 3-d representations 9-10 Calculating the probability of finding the ewithin volume element dV is proportional to 2 Total energy EF have n-l-1 radial and l angular nodes Example Problem 9.3 Locate the nodal surfaces in The angular part, cos, is zero for =/2. In 3D 9-11 The radial part of the equations is zero for finite values of for This occurs at r= 0, and at r= 6 a0. The first value is a point in three dimensional space and the second is a spherical surface. In an atom, the probability of finding e-at certain distance, r, from the nucleus is of more interest than finding it in dV. Define radial probability distribution function (rpd) as P(r)dr P(r)dr gives the probability of finding the electron in a spherical shell of radius rand thickness dr. 9-12 Example for 1s orbital. Note that rpd function goes to zero as r goes to zero 9-13 In Quantum mechanics we draw probability density map instead of the “planetary” model 9-14 Example Problem 9.6 Calculate the maxima in the radial probability distribution for the 2s orbital. What is the most probable distance from the nucleus for an electron in this orbital? Are there subsidiary maxima? Plot P(r) and Principal maximum in P(r)is at 5.24a0. Subsidiary maximum is at 0.76a0.