Quantum Mechanics and Electron Configuration Problems Solutions
advertisement

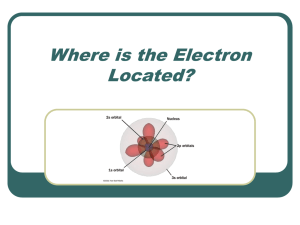
Quantum Mechanics and Electron Configuration Problems Solutions First: n = 1,2,3,4 ... infinity l = 0,1,2,3 .. (n-1) (to specifiy l values you have to know which n you are talking about) ml = -l, (-l+1) .. 0 .. (l-1), +l (to specifiy ml values you have to know which l you are talking about) ms = +1/2 or -1/2 also: if if if if l l l l =0 this corresponds to an s-orbital =1 this is a p-orbital = 2 this is a d-orbital = 3 this is an f-orbital 1. For n = 4, l can take four possible values, 0,1,2,3 For l = 2, ml can take five possible values, -2, -1, 0, 1, 2 2. a) Impossible. If n = 1 the only allowed value of l is 0. In other words, 1s is the only possible orbital with n = 1. b) ok. The 4s orbital can exist since the rules for combinations of quantum numbers allow n = 4 and l = 0. c) ok. The required quantum numbers for a 5f orbital are n=5, l=3 (plus an ml value for each of the seven possible degenerate orbitals) d) Impossible. If n = 2, l can be 0 or 1. A d-orbital corresponds to an orbital with an l value of 2. 3. a) this set of quantum numbers is ok and obeys the above rules. b) this set is not ok because if l =0 the only possible ml value is 0. c) this set is ok d) this set is not ok since if n = 3 the highest possible l value is 2. a) [Xe] 6s1 b) [Ar] 4s23d8 c) [Ar] 4s23d104p4 d) [Kr] 5s24d10 e) [Rn] 7s26d1 f) [Xe] 6s24f145d106p2 5. [Rn] 7s26d105f147p6 This element will be part of the noble gas group of elements. 6.a) This is not a ground-state electron configuration .. the electron in the 3s orbital should be in a 2p orbital b)[Ne] implies a completely filled n=2 level. The next few electrons will fill the 3s and 3p orbitals. c)Not a ground-state electron configuration. The electrons in the 3d orbitals should be in the lower energy 3p orbitals. 7.a) Nitrogen, 1s22s22p3 b) Selenium, [Ar] 4s23d104p4 c) Rhenium, [Kr] 5s24d7 8. If there were three possible spin quantum numbers for the electron then it would be possible to put three electrons into an orbital rather than two. Thus, the s-block of elements in the table would be three elements wide instead of two. The p-block would be 9 elements wide and the d-block would be 15 elements wide. Hydrogen and helium would be s-block elements and lithium would be at the top of the noble gas group. Berylium, boron and carbon would be period 2 s-block elements and the period 2 p-block elements would consist of nitrogen to phosphorus, phosphorus being the next noble gas. The chemistry of elements would be utterly different!