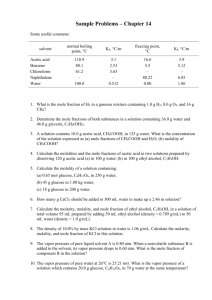
kr,,,o*,'
advertisement

Chemistry
11
Solutions.
Honework2
Tf
Sect.+
.t,
(l) A solution is madeby dissolving 25.88 g urea (CIIaN2O),a nonelectrolyte,tn275 gwat$.
Calculatethe vaporprcssuresof this solution at 25"C and 45t. The vapor pressureof water is
23.8 totr at 25"C and71.9 torr at 45"C.
,
a(Hr4
n(Curttr\, L{.tl/eo.r,t> o.'l\D, 6(
( (roc , ?' J+
Y--
tt.,*-t
, L\:1",, ,( D.i]1)(&,t),7;..1
lo17
- _:-
E.a+0}1\D
Q qf. , P. (o.nrJ(rr.l)= Lt.1lo,,
(2) A solution is madeby mixing 50.0 g of acetone(CH3COCH:)and 50.0 g of methanol
(CEOI{). What is the vaporpressureof this solution at 25qC?What is the compositionof the
vapor expressedas a mole fraction? At 259Cthe vaporpr,essures.
of aqetoneand methanolare .
271 and 143 totr, respectively. The actualvapor pressureof the solution is 161 torr. Explain any
discrepa4cies.
n(r,!,*J = ty
=
7. o.gLL,n,1 n(a.rrow)
3z.D I
{i.oa
b.frq-
kr,,,o*,'6 . 1 c 2
Prorr*,
/rur*,,=
.i
6 t (
.J
.--
b.{tL.l.u
=
tal
| 14"t
.
G
/
1/
/'c{fll'
-
[.sL,*.r
ka\Dtl= l-Ta,or\= b.L'/4
= 03{L
+ l.fL
a v?''
ro,u ,
| nt
'rcrrr*ry= o.,tf?
4",,
-__->
t
L<t5,- <,,.o'tz'l '.[ igg
bun, ?r,o=) (-) dr,tulu;n
ir
:_:
(3) Which of the following will havethe lowest total vapor pre'ssureat 25"C?
a. waler
b. an aqueousglucosesolution with a mole fraction of glucose= 0.01
c. an aqueoussodium chloride solution with a mole fraction of NaCl = 0.01
d. a solution of metlnnol in water with mole ftaction of methanol= 0.2. The vapor
pressureof methanolat 25'C is 143 torr.
tr{
Alaclort( )issoc'tl.,
1. cr4, ,^otL i., s,1^{,;.
tAA.
(4) A solution contains3.75 g of a nonvolatile pure hydrocarbonin 95 g acetone.The boiling
points of pure Bcetoneand the solution are 55.95 and 56.50t, respeptively. .Themolal boilingpoint constantof acetoneis 1.71'C kg/mol. What is the molar massof the hydrocarbon?
kf6' o.rd"<
LTi, kt u+l solulz
o
A -'Z
KI
|
,
fta Saluaat
J
,r'tr- 5ol,4r :
kt
ttr<st go\ul:-
l1sof,..*{4T
"
Ma(l 5olrl
l4q i(-l-
\ sob,^4
= (t-tr)lr.rr)
= 113f/
(o.orr)1o.rs)*1
(5) Calculatethe freezing point and boiling point of an antifreezesolution that is 40-07oby mass
of ethyleneglycol (HOCHzCItrzOI{)in water. Ethylene glycol is a nonelectrolyte.
looS
tolJ,*. 4 ,lo.oj 5l*l t Lu"oJH,,
n(3lyr"t)
' ' t o ' o / t r . t ' 6 . 1 4 { , - l n(5Q-t)
"
Nt "(t t4 (rnru): Lt.o at , -1,0,o
L
......-.--.---.-
Mr (0.r,fu,.+u),
(.,if
7, lodr".
..-...>
:Y1-
6.oL"b
: to.?,t-,-
(6) Anthraqufuonecontainsonly carboq hydrogen,and oxygen and hasan empirical formula of
C*I4O. The freepingpoint of gq4plor is lowetsd by ?2.3"Cwhelr 1.32g a4hraqni4ore.is
dissolvedin 11.4g camphor. Deterndnethe molecula formula of mthraquinone.
Kr(camphor)= 4O.0"C kg/mol.
Alna' k4. o,ou
-...-
AT4.
&5
- ( t o. o )(t:z )
: UY Jf^"1
/zze)1o."ttr)
:> -l'''u'o'(
6^f ,.,i.\ F*al.' to4Jl,^ot
(7) How would you pre.pare1.0 L of an aqueoussolution of sodiumchloride having an.osmotic
pressureof 15 atm *.2TC2
14'c--TT 3
t{
-,----
Rr (r.,ru)(zr)
:
O.LL ^11,
n (il"u) = D-* = o.\t ,,^."1
; tt.tS Mc1
1Dirtol* tt.j u<<ti,,, /.t,L ilro
(8) Placethe following solutiors in order by the size ofthe freezing-pointdepression.
a. 1.0 m glucosein water (glucoseis a molecular solid)
b. 1.0 m NaCl in water
c. 1.0 m HOCI in water (HOCI is a weak acid)
d. 1.0 MgCl2 in water
q < < <T b < a
(9) Formic acid (HCOzH) is a weakmonoprotic acid. A 0.10 M formic acid solution is 4.27o
ionized. Assumingthat the molari.ty and molality of thq solution arethe same,calcqlatethe
point of 0,10 M formic acid
freezing point and the boiling
*'""1'**':rvrrur''*-de.'
HcorHe
f H+7
T;*_r,/oo
- 'b
: 't.L (HuruJo.D.ruD
H++ @*t'
fitrJ=fr"f] = o.oxttLtt
: ol>l{7
ln.qerJ: 6.ro-6,oot{L
(t"+^t solltJ :
6.oD.1L
+ o.o.tlL1d.offt; 6.r6LlLM
ATf =
lt.tu)(o.o<t-;: o.L6bc
(10) A solid consistsof a mixture of NaNO3and Mg(NO:)2. When 6.50 g of the solid is
dissolvedin 50.0 g water, the freezing point is lowered by 5.4trC. What is the composition,by
mass,of the soljd?
Ai
t#,
*.
({
-
r.tLo<f
*.
r-'10,u.*1^6.oso(,:
6J
6l,1g-ul5,1.1..
,:n
4oroh, hlu<^4
'Tok(,,,
-t tol,tlz: ^ : L(X*,,tN"rvor)
+ t(J ^. I tt1fuor),)
: 6./4{^^,1
Tll a^*ss,l'\.z L\ ,
(rr" J/^,,)ft) r(/$x/"*,)
(r)
( t '
Jttth.t
5"tJl*,
jt,unll'-\ntur\
,'i,o
U : D . 6l l
J
---\
2,11t<1(uor),Q
",rl Y.tJ
r
o
Na t\)t
J
-,,1 tu\1(u)_
(11) Is soapa more effective cleaneris freshwateror in qeawater?Explain.
!o,7 $,*-, tolloqlparh'l"s lln"l ["c't' 0..'\uf' !urI,"t'.1ru. ,a.[J.t L,u^
r-!ul
fi*;,|..r clnrryt tb o^. to(6',.y'
1"rl,d- d>rilpp.1"^olL*. In
..--\''#l
s.1,+ol..1JL,;^H u',ll cqotr lL) dh,t p,,l,.L ]o t,a5olol..
'74'o'
o{ to[JrLlLr""\ ttilu*t) lh, o,i e{ftel,n<,.rss.
i s,llt-o4
(12) The phasediagramof water discussedin the previousunit describesthe phasechanges
when only water is present. Considerhow the diagramwould changeif air were also presentat 1
atm and dissolvedin the water.
a. Would the new fiple point temperaturebe higher, lower or the sameas the triple point for
pure water? Explain.
J,n,,ll," Prrr{t"L*^1,P",prA lL Tf {ay. v"-t/ 6, louo.
b. Would ice sublime at a fery degreesbelow the fteezing point underthis pressure?Explain.
oo"ll'
v I
Iet
bt,-+otj,^rL [tJr- lL, n^ l.,tr, yr,l.
c. Would the liquid havethe samevapor pressureat 100"Cas that for pure water?
/lo Luour^lL fry L:oau 6c LrnJ
t+ 1" IL" "l,s,,Lrl dr.
(13) It hasbeenmentionedthat the valuesfor molality and molarity arenearly the samefor
dilute aqueoussolutions. Is this true for nonaqueoussolventsaswell? Explain.
1/
ao^Ll l" tnu. k
all to*",|t uLo,.- Jznti\
tl
t
f
cloSc- |t
(.6
Slnt
t
v c (al
![Cindy Cai Ch. 10 & 24 gas & complex ion Rx test [50 points] AP](http://s3.studylib.net/store/data/009017289_1-2d8fa1d50414889bc159ab8c8d265314-300x300.png)