Cindy Cai Ch. 10 & 24 gas & complex ion Rx test [50 points] AP
advertisement
![Cindy Cai Ch. 10 & 24 gas & complex ion Rx test [50 points] AP](http://s3.studylib.net/store/data/009017289_1-2d8fa1d50414889bc159ab8c8d265314-768x994.png)
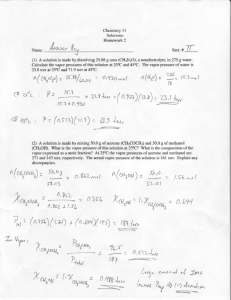
Cindy Cai Ch. 10 & 24 gas & complex ion Rx test [50 points] AP Chemistry 1. Give the formulas to show the reactants and products for the following chemical reactions. Each occurs in aqueous solution unless otherwise indicated. Represent substances in solution as ions if the substance is extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. In all cases a reaction occurs. You need not balance (the coefficients in the reactions). Pick 5 problems, only. [15 points] a. mix solutions of iron (II) chloride and a concentrated solution of ammonia FeCl2 + NH3 FeNH3Cl b. sodium metal reacts with oxygen gas 4Na + O2 2Na2O c. excess sodium cyanide solution added to a solution of silver nitrate NaCN + AgNO3 AgCN + NaNO3 d. mix solutions of sodium sulfide and zinc fluoride Na2S + 2ZnF 2NaF + Zn2S e. an acidic solution of potassium permanganate reacts with silver 8H+ +MnO4- + 5e- Mn+2 + 4H2O 5(Ag Ag+ + e-) 8H+ +MnO4- + 5Ag Mn+2 + 4H2O + Ag+ f. copper (II) hydroxide reacts with excess potassium cyanide solution 4 Cu + 8 KCN + 2 O2 + 2H2O → 4 K[Cu(CN)2] + 4 KOH Cu + 2 CN- +2 O2 + 2H2O [Cu(CN)2]-2 + 4 OHg. copper metal in dilute nitric acid Cu Cu+2 + 2eH+ + NO3- + 2e- NO + 2OHCu + H+ + NO3- Cu+2 + NO + 2OHFor problems involving calculations, show your work in an organized manner, includes any relevant formula / equation, the proper units in your work and answer, and the proper number of significant figures in your answer. 2. 100. mL 250. mM HCl reacts with 1.0 g Mg at 26◦C (where PH2O = 25 torr). Hydrogen gas was collected over water, where the height of water inside and outside of the container was equal (i.e. Pgas = Patm = 1.0 atm). [15 points] a. # moles of H2 produced = __. 2HCl + Mg H2 + MgCl2 ( ( ) = 0.025 mole HCl limited 1g = 0.041 Mg 0.025 mole HCl = 0.0125 mole H2 b. # mL H2 produced = __. PV = nRT V= = = 0.307 L = 307 mL c. # mL H2 produced at STP = __. @ STP: 22.4 mole = 1 L 0.0125 mole H2 = 0.558 mL 3. 2.0 mL of an unknown compound was completely vaporized in a 125 mL flask immersed in a 90◦C water bath, then the flask was put into a 4◦C water bath (to condense the vapor), where 193 mg of the compound was recovered. Patm = 760 torr. Determine the molar mass of the compound. [10 points] Molar mass = n= = = 4.19 molar mass = mole unknown = 46.01 4. A 250. mL sample of gas at 25◦C and 700 torr was cooled to 4◦C and the volume reduced to 100. mL. What is the new pressure in the container ? [10 points] = = P2 = 1627 torr