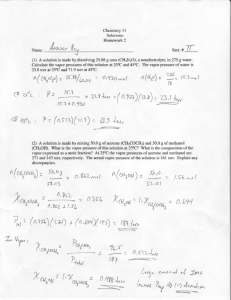
CHEM 341. Fall 2000. Problem Set #7. 1. At 35
advertisement
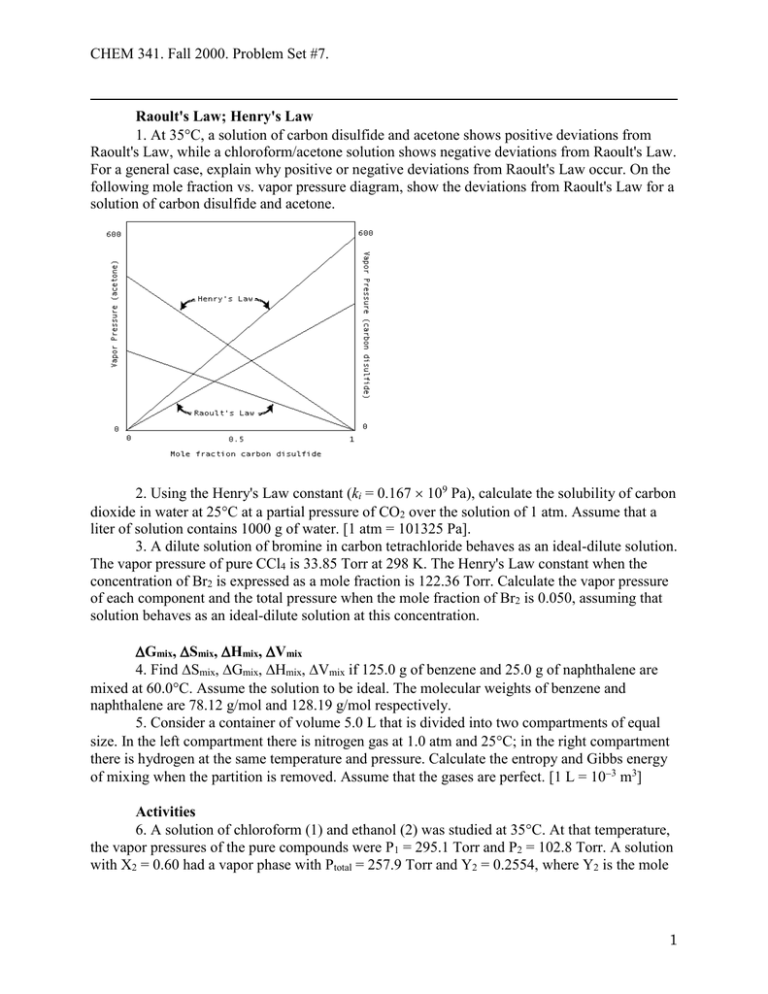
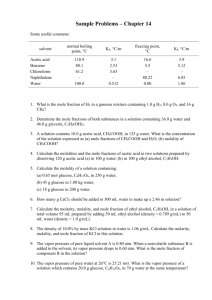
CHEM 341. Fall 2000. Problem Set #7. Raoult's Law; Henry's Law 1. At 35C, a solution of carbon disulfide and acetone shows positive deviations from Raoult's Law, while a chloroform/acetone solution shows negative deviations from Raoult's Law. For a general case, explain why positive or negative deviations from Raoult's Law occur. On the following mole fraction vs. vapor pressure diagram, show the deviations from Raoult's Law for a solution of carbon disulfide and acetone. 2. Using the Henry's Law constant (ki = 0.167 109 Pa), calculate the solubility of carbon dioxide in water at 25C at a partial pressure of CO2 over the solution of 1 atm. Assume that a liter of solution contains 1000 g of water. [1 atm = 101325 Pa]. 3. A dilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. The vapor pressure of pure CCl4 is 33.85 Torr at 298 K. The Henry's Law constant when the concentration of Br2 is expressed as a mole fraction is 122.36 Torr. Calculate the vapor pressure of each component and the total pressure when the mole fraction of Br2 is 0.050, assuming that solution behaves as an ideal-dilute solution at this concentration. Gmix, Smix, Hmix, Vmix 4. Find Smix, Gmix, Hmix, Vmix if 125.0 g of benzene and 25.0 g of naphthalene are mixed at 60.0C. Assume the solution to be ideal. The molecular weights of benzene and naphthalene are 78.12 g/mol and 128.19 g/mol respectively. 5. Consider a container of volume 5.0 L that is divided into two compartments of equal size. In the left compartment there is nitrogen gas at 1.0 atm and 25C; in the right compartment there is hydrogen at the same temperature and pressure. Calculate the entropy and Gibbs energy of mixing when the partition is removed. Assume that the gases are perfect. [1 L = 103 m3] Activities 6. A solution of chloroform (1) and ethanol (2) was studied at 35C. At that temperature, the vapor pressures of the pure compounds were P1 = 295.1 Torr and P2 = 102.8 Torr. A solution with X2 = 0.60 had a vapor phase with Ptotal = 257.9 Torr and Y2 = 0.2554, where Y2 is the mole 1 CHEM 341. Fall 2000. Problem Set #7. fraction of ethanol in the vapor phase. Calculate the activity and activity coefficient of ethanol in this mixture. Colligative Properties 7. Calculate the freezing point of 250 cm3 of water containing 7.5 g of sucrose. [Kf = 1.86 K kg mol1; 1 cm3 water = 1 g; the molecular weight of sucrose is 342.3 g/mol.] 8. A sample of myoglobin with mass 1.00 g is dissolved in water to make 100.0 mL of solution. The osmotic pressure of the solution was 11.0 Torr at 25.0C. Find the molar mass of myoglobin. Clapeyron Equation 9. Calculate the change in pressure required to change the freezing point of water 1C. At 0C the heat of fusion of ice is 333.5 J g1, the density of water is 0.9998 g cm3, and the density of ice is 0.9168 g cm3. [1 m3 = 106 cm3] 10. The change in enthalpy is given by dH C p dT VdP . The Clapeyron equation relates dP and dT at equilibrium, and so in combination these two equations can be used to find how the enthalpy changes along a phase boundary as the temperature changes and the two phases H remain in equilibrium. Show that d C p d ln T . T Clausius-Clapeyron Equation 11. Naphthalene, C10H8, melts at 80.2C. If the vapor pressure of the liquid is 10 Torr at 85.8C and 40 Torr at 119.3C, use the Clausius-Clapeyron equation to calculate the enthalpy of vaporization at 40 Torr; and the normal boiling point. Chemical Equilibria 12. The equilibrium constant of the reaction 2C3 H 6 g C2 H 4 g C4 H 8 g is found to fit the expression 1088 1.51 105 ln K 1.04 T T2 between 300 K and 600 K (T is measured in K). Calculate the standard reaction enthalpy and standard reaction entropy at 400 K. 13. What is the standard enthalpy of a reaction for which the equilibrium constant is doubled when the temperature is increased by 10 K at 298 K? 2