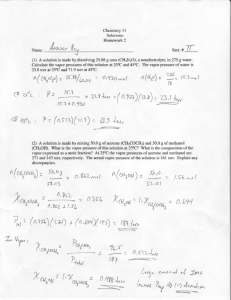
Use the DePriester chart to generate the temperature
advertisement
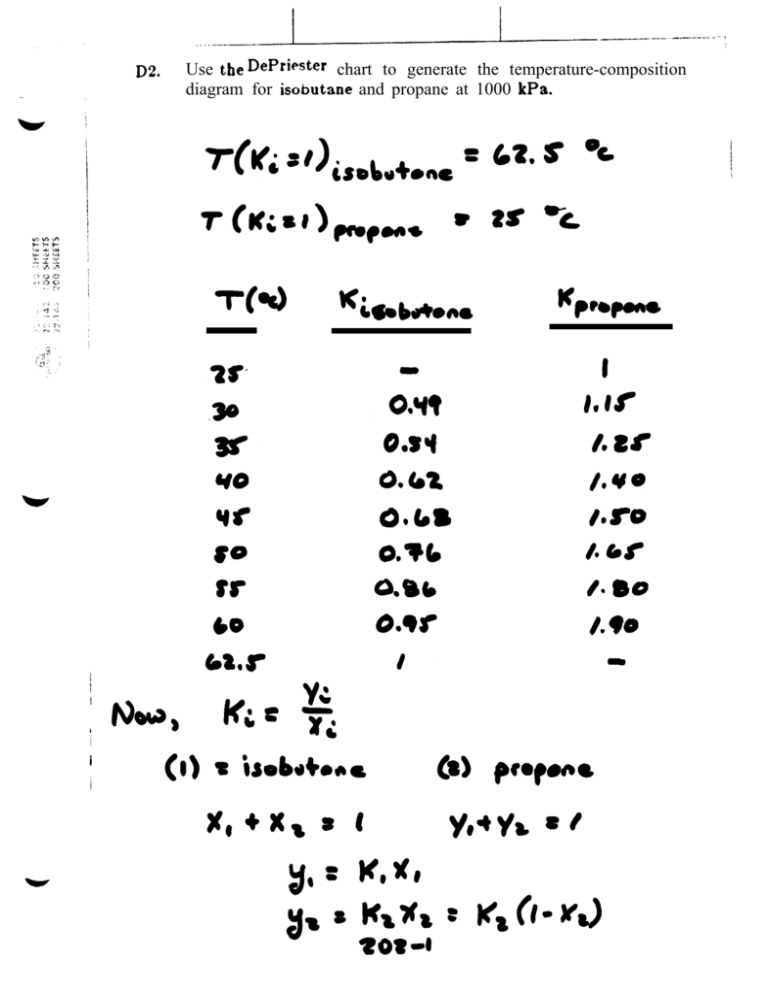
. . . . ..--~--— ---------- ~~~ D20 Use the DePriester chart to generate the temperature-composition diagram for isobutane and propane at 1000 kPa. T(Q I I I I , -— --- . . --- I= I . I , o,~$o 0.?s0 e. $s I V8 —— D4. .- —.- ---- -. . . If a 40 mole % ethanol, 60 mole % water mixture at 60° C and 1 atm is heated: ——————- a. At what temperature does it first begin to boil? What is the composition of the first bubble of vapor? b. At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last dropIet of liquid? c. At 82° C, what fr=tion is liquid? d. When 80% has been vaporized, what iS the temperature and what are the liquid and vapor compositions? t ! c) I I I ..* *n ,.- *. *I ,,, r,,,, . . L,, :. I r z-oq-2 I I , [ I , I .... ~.~- . . . . . . . . . . . . . . - D1l. A t 1 atm, the ethylene dibromide-propy lene dibromide system has a constant relative volatility of a= = 1.30 (Perry et al., 1963, p. 13-3). Use the aEP value to generate the y-x equilibrium diagram. I Ye - Ye I I (2-2s] I i -------------- $ , I /0 9 2D11 Page 1 . E2. Uw +be O* W;ehf b) , I I ______ -- Find the dew-point and bubble-point temperatures for a mixture that is 20 mole ~o n-butane, 50 mole % n-pentane, and 30 mole % n-hexane. Pressure is 1 atm. a) > ..__.~ ~Sc ~$, (~m~~) , C&d& ! u- .. ,. ,’ .: Vo 0. s“ /, ..-. — , . . . ,! .’ .’ I .: I II I I [ t —— - ( Problem 2E2 At2 Atl At6 -1280557 -1524891 -1778901 butane pentane hexane Apl Ap2 7.94986 7.33129 6.96783 o 0 0 Ap3 -0.96455 -0.89143 -0.84634 o 0 0 p (psia) = 14.696 p (psia) = 14.696 Bubble Point Calculation T (0 C)= 27.8 541.66 0 T ( R)= 0.2 0.5 0.3 butane pentane hexane Ki*zi K zi 2.70 0.77 0.25 0.54 0.38 0.08 1.00 Dew Point Calculation T (0 C)= T (0 R)= K zi butane pentane hexane 0.2 0.5 0.3 46.4 575.2 zi/Ki 4.42 1.39 0.50 0.05 0.36 0.59 1.00 Page 1 0 0 0 I .-.L—- -.., .-. . . D9. ! .- .—--+ ., A mixture of n-butane, n-pentane, and n-hexane is at 120° F and 20 psia. Liquid and vapor are in equilibrium. If the liquid is 0.10 mole fraction h-butane, find the compositions of Iiquid and vapor. Note: Watch your units. This problem is not trial-and-error. I ! , I I Kcb = . . . . . . . . . . . . . . . . . . . ._, -, ,“-,, ,... ~.... I