Vapor Pressure Lowering Example Problem
advertisement

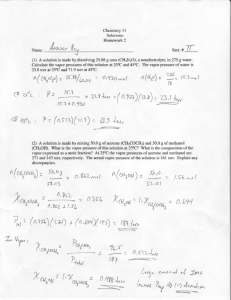
Kuwata Chemistry 311 Fall 2007 Chapter 5 Example Problem—Vapor Pressure Lowering The vapor pressure of (pure) water at 20.0°C is 17.535 Torr. Consider a solution in which 30.00 g of lithium sulfate (M = 109.95 g mol-1) is completely dissolved in 500. g of water (M = 18.02 g mol-1). Assume that Raoult’s Law applies to the solution. (a) (b) (c) Predict the vapor pressure of the aqueous lithium sulfate solution at 20.0°C. Careful measurements with an isoteniscope reveal that the actual vapor pressure of the aqueous lithium sulfate solution at 20.0°C is 17.074 Torr. What is wrong with our calculation in part (a)? What is wrong (or at least overly simplistic) with our refinement in part (b)?