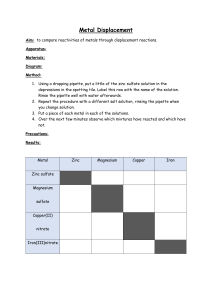
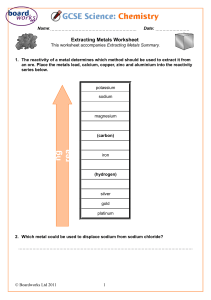
Form 4 Chemistry Exercise
advertisement
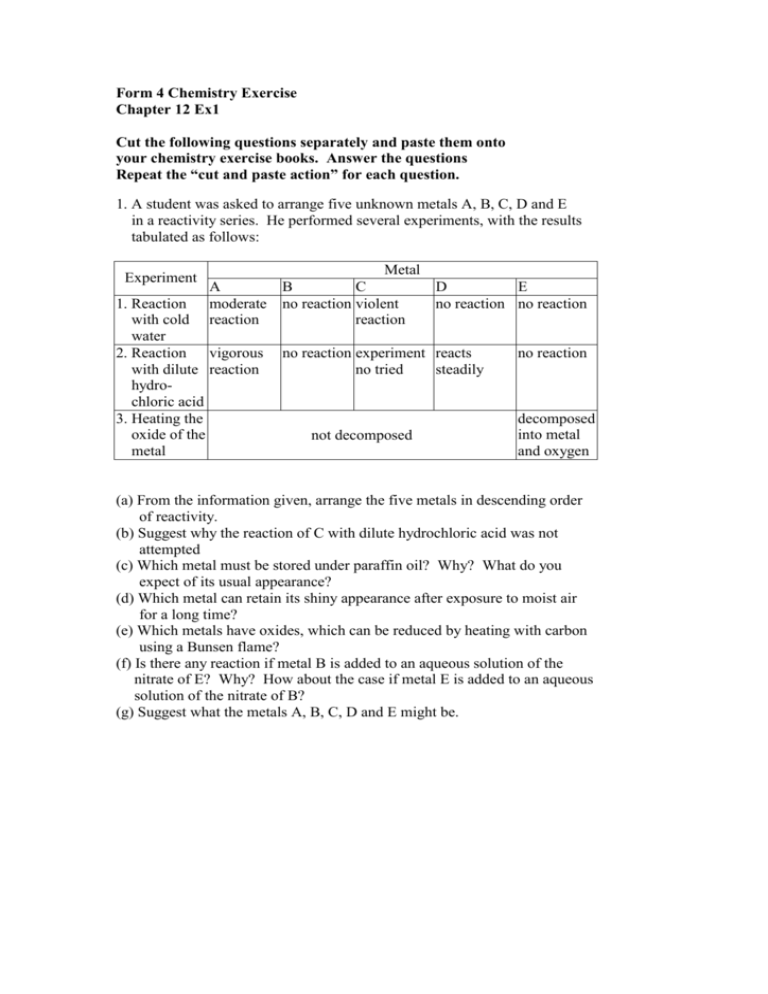
Form 4 Chemistry Exercise Chapter 12 Ex1 Cut the following questions separately and paste them onto your chemistry exercise books. Answer the questions Repeat the “cut and paste action” for each question. 1. A student was asked to arrange five unknown metals A, B, C, D and E in a reactivity series. He performed several experiments, with the results tabulated as follows: Experiment A moderate reaction 1. Reaction with cold water 2. Reaction vigorous with dilute reaction hydrochloric acid 3. Heating the oxide of the metal Metal B C D E no reaction violent no reaction no reaction reaction no reaction experiment reacts no tried steadily not decomposed no reaction decomposed into metal and oxygen (a) From the information given, arrange the five metals in descending order of reactivity. (b) Suggest why the reaction of C with dilute hydrochloric acid was not attempted (c) Which metal must be stored under paraffin oil? Why? What do you expect of its usual appearance? (d) Which metal can retain its shiny appearance after exposure to moist air for a long time? (e) Which metals have oxides, which can be reduced by heating with carbon using a Bunsen flame? (f) Is there any reaction if metal B is added to an aqueous solution of the nitrate of E? Why? How about the case if metal E is added to an aqueous solution of the nitrate of B? (g) Suggest what the metals A, B, C, D and E might be.