Metal Reactivity Lab Report: Activity Series & Periodic Trends
advertisement

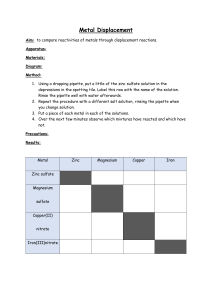
Metal Reactivity Lab I. Purpose: Determine if a relationship exists between element placement on the periodic table and its stability (reactivity). II. Materials Metals: copper, zinc, aluminum, magnesium, iron, and lead Solutions: 2 M hydrochloric acid (HCl), copper (II) chloride (CuCl2), silver nitrate (AgNO3) Equipment: spot plate, stirring rod, test tube brush, waste beaker for cleaned metals III. Procedure: Write a detailed procedure so that any logical person could repeat your experiment. Make sure your procedure is in the proper format. IV. Data – All data must be in a table. Make sure it is nice, neat, and easy to read (ruler edges or formatted, titled, labeled, and includes all experimental data). Leave a space for writing the balanced chemical reaction. V. Analysis: Use your data to answer the following questions. Answer using complete sentences while incorporating question. Questions: 1. What were the metals used in this lab? List all solid metals and those in solution, including hydrogen. 2. What types of phenomena in this lab indicated that a chemical reaction (chemical change) was taking place? Include all that apply. 3. How can you determine which metal was more reactive? Be specific. 4. Place your metals in an activity series (order from most reactive to least reactive). 5. How does your reactivity series compare to other groups? Compare your results with 2 other lab groups and state differences in results. 6. In analyzing YOUR DATA, is there any relationship with the placement of the metal on the periodic table and its reactivity? Consider both down and across a row. Is there a general statement you could make? If your answer is “Yes,” write your general statement. Either way, explain why you answered the way you did. This question is about analyzing your data (not about book answers or others work). VI. Conclusions Write a paragraph discussing the purpose of the lab and if you achieved this purpose. Explain why or why not. Give support for your statements by citing examples from your lab/observations. Explain controllable and uncontrollable errors that may have contributed to any deviations in your results. State how these errors affected the results. Also, explain what you would do differently to avoid any deviations. *Think through this paragraph and make it quality work! Student Name:________________________________________ Period __________ Lab:____________________________________ Metal Reactivity Lab POINTS Standard Lab Report Rubric CRITERIA Title (1) Your name & partner(s), correct title, & date given I. Purpose (1) States hypothesis or objective for lab. II. Materials (2) List of all correctly named materials needed to perform lab. III. Procedure (5) Present information in sequential order. Clearly written for exact replication – no clarifying questions needed. Refers to what data needs to be collected. IV. Data (6) Titled Neatly organized using ruler or formatted table. All data (qualitative and/or quantitative) included. All categories labeled (horizontally and vertically) Observations/data are detailed and specific V. Analysis (Questions) (14) Use complete sentences incorporating question 1. 2. 3. 4. 5. 6. VI. Conclusions/Analysis (11) Restate purpose of lab and whether met purpose. Explanation as to why / why not. Support statements from lab. Describe sources of error and their affects. State improvements or future variables if you were to repeat lab. Format (2) Typed or neatly written, correct spelling, correctly organized. SCORE Teacher Comments: 0 1 2 3 4 / 40 5