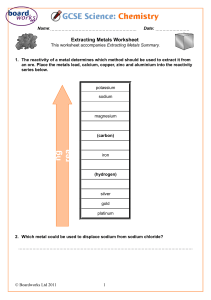
Metal Displacement Aim: to compare reactivities of metals through displacement reactions. Apparatus: Materials: Diagram: Method: 1. Using a dropping pipette, put a little of the zinc sulfate solution in the depressions in the spotting tile. Label this row with the name of the solution. Rinse the pipette well with water afterwards. 2. Repeat the procedure with a different salt solution, rinsing the pipette when you change solution. 3. Put a piece of each metal in each of the solutions. 4. Over the next few minutes observe which mixtures have reacted and which have not. Precautions: Results: Metal Zinc sulfate Magnesium sulfate Copper(II) nitrate Iron(III)nitrate Zinc Magnesium Copper Iron Discussion: • Place the metals Mg, Cu, Zn and Fe in order of reactivity using the results obtained from your experiment. • The reactivity series also controls the reactivity of these metals with acids. Which metals from those used in the experiment would react with hydrochloric acid? Which metals would not react with hydrochloric acid? And why? • The reactivity series also determines the extraction of metals. Use the reactivity series you have obtained, and state which method of extraction would be used for every metal. • How can the method be improved?