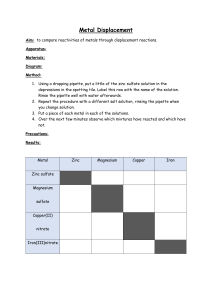
Name:……………………………………………………. Date: …………………… Extracting Metals Worksheet This worksheet accompanies Extracting Metals Summary. 1. The reactivity of a metal determines which method should be used to extract it from an ore. Place the metals lead, calcium, copper, zinc and aluminium into the reactivity series below. potassium sodium magnesium Incr easi ng rea ctiv ity (carbon) iron (hydrogen) silver gold platinum 2. Which metal could be used to displace sodium from sodium chloride? …………………………………………………………………………………………………………… © Boardworks Ltd 2011 1 Name:……………………………………………………. Date: …………………… 3. Name two metals that can only be extracted by electrolysis. …………………………………………………………………………………………………………….. …………………………………………………………………………………………………………….. 4. Name a metal that could be extracted from its ore using carbon. …………………………………………………………………………………………………………….. 5. Suggest a reason why iron is extracted using carbon rather than by electrolysis. ……………………………………………………………………………………………………………. ……………………………………………………………………………………………………………. ……………………………………………………………………………………………………………. ……………………………………………………………………………………………………………. 6. Suggest a reason why gold is expensive, even though it is found native in rock. ……………………………………………………………………………………………………………. ……………………………………………………………………………………………………………. ……………………………………………………………………………………………………………. …………………………………………………………………………………………………………… ………………………………………………………………… © Boardworks Ltd 2011 2