Section 3 Notes
advertisement

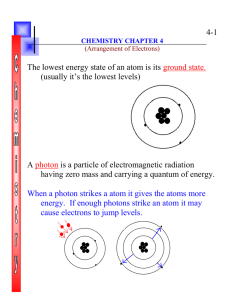
«F_Name» «L_Name» Period «Per» Chapter 4 Section 3 «Num» Chapter 4 Sec 3: Rules Governing Electron Configurations Pauli Exclusion Principle states that no two electrons in the same atom can have the same four quantum numbers. Every electron is different than all the other electrons in an atom. No 2 electrons can be in the same space Electron Configurations: the arrangement of electrons in an atom. An arrangement – the “certain way” the electrons are together Configuration Notations: The model used to exhibit electron configurations. Electron arrangement written down Aufbau Filling Principle An electron occupies the lowest orbital (energy) that can receive it. Energies are filled from the bottom up Hund’s Rule states that orbitals of equal energy are each occupied by one electron before any are occupied by a second electron and all electrons in singly occupied orbitals must have the same spin. all orbitals in a group get one electron before any get 2; all first electrons have the same spin direction Examples of the p-orbital filling This figure shows how two (a), three (b), and four (c) electrons fill the p sublevel of any given main energy level according to Hund’s rule. Since up and down are arbitrarily assigned, convention has given that we start with up and then down after the “up spin” has filled a sublevel. sublevel = orbital orbital notation examples: «F_Name» «L_Name» Period «Per» «Num» «F_Name» «L_Name» Period «Per» «Num» Orbital Notation In Orbital Notation, an unoccupied orbital is represented by a line, with the orbital’s name written underneath the line – the next step after the Bohr model _______ 1s An orbital containing one electron is represented as two electrons is represented as opposite spins. . An orbital containing , showing the electrons paired and with Putting it all together: The lines are labeled with the principal quantum number and sublevel letter beneath the line. For example, the orbital notations for Hydrogen, Helium, and Boron are written as follows. Hund’s rule is followed when using this notation. For Example Oxygen’s notation is: Electron-configuration notation eliminates the lines and arrows of orbital notation. Instead, the number of electrons in a sublevel is shown by adding a superscript to the sublevel designation. Hydrogen’s Electron-configuration notation is 1s1. The superscript indicates that one electron is present in hydrogen’s 1s orbital. Helium’s configuration is 1s2. The superscript indicates that there are two electrons in Helium’s 1s orbital. «F_Name» «L_Name» Period «Per» The electron configuration for Boron is 1s22s22p1. The superscript indicates the number of electrons in each orbital. The electron configuration for Oxygen is 1s22s22p4. The superscript indicates the number of electrons in each orbital. Electron-configuration notation general Rule First write the Principle Quantum number (the energy level: same as the period on our periodic table). Then write the angular momentum quantum number (the orbital shape: s, p, d, or f) Finally, superscript the number of electrons in the orbital. (maxes: s 2, p6, d10, and f14) n l # of electrons in the orbital Principle Quantum Number: n (1 – 7) Angular Momentum Quantum Number: l (s, p, d, or f) # of electrons in the orbital (just the e –’s in the “l” orbital «Num» Coefficient is the principle quantum #; the letter is the angular momentum quantum #; the superscript is the number of electrons, this indicates both the magnetic quantum # and spin «F_Name» «L_Name» Period «Per» Nobel Gas Configuration Neon’s electron configuration is 1s22s22p6 Phosphorus’s electron configuration is 1s22s22p63s23p3 Notice how Neon’s configuration is the first part of Phosphorus’s configuration. Neon 1s22s22p6 Phosphorus 1s22s22p63s23p3 Just like you can substitute in a math equation, chemist substitute Neon’s configuration with Neon’s symbol Square bracketed to distinguish that it is Neon’s configuration and not Neon. Similarly, all the noble gas configurations can be used to substitute into configurations as shorthand. P’s Nobel - Gas Notation: [Ne] 3s23p3 This shorthand configuration is called Nobel - Gas Notation. See page 111, 112, and 114 for more examples. «Num» «F_Name» «L_Name» Period «Per» «Num» Inner-Shell Electrons: electrons that are not in the highest occupied energy level. Highest Occupied Level: the electron-containing (has electrons in it) main energy level with the highest principal quantum number. Draw Orbital Notation, Electron Configuration Notation, and Nobel - Gas Notation for fluorine, chlorine, bromine and iodine. (The halogens) F: 1s2 2s2 2p5 F: [He] 2s2 2p5 Cl: 1s2 2s22p6 3s23p5 Cl: [Ne] 3s23p5 Br: 1s2 2s22p6 3s23p63d10 4s24p5 Br: [Ar] 3d10 4s2 4p5 I: 1s2 2s22p6 3s23p63d10 4s24p64d10 5s25p5 I: [Kr]4d105s25p5 Also a note about the Halogens and a few other elements of the Periodic Table: There are some elements that do not exist as single atoms in their elemental form. They exist as “pairs” and are called Diatomic elements The diatomic elements are hydrogen, nitrogen, oxygen, and the halogens: fluorine, chlorine, bromine, iodine, and astatine. Astatine is so rare in nature (its most stable isotope has a half-life of only 8.1 hours) that it is usually not considered. Many metals are also diatomic when in their gaseous states. HW: