Cyclohexene Synthesis: Green Chemistry Lab Experiment
advertisement

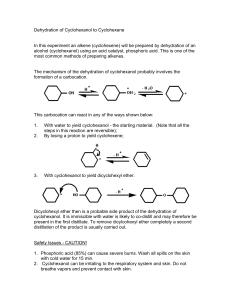
Chemistry 241 Exp 7. Cyclohexene from Cyclohexenol To read more about green chemistry, try: http://www.rsc.org/Publishing/Journals/gc/index.asp Dehydration reactions: http://dl.clackamas.cc.or.us/ch106-03/intramol.htm Separatory funnel use: http://orgchem.colorado.edu/hndbksupport/ext/extprocedure.html Drying agents: http://orgchem.colorado.edu/hndbksupport/drying/drying.html http://www.brynmawr.edu/Acads/Chem/mnerzsto/drying_agents_1999.htm This synthesis reaction is the reverse of the hydration addition reactions that we are studying now in chapter 4. As a matter of fact, every step in this reaction is potentially reversible. We will use Le Chatelier’s principle and remove the cyclohexene as it is formed to drive this reaction towards products. This reaction is green in two respects. First, it uses phosphoric acid instead of sulfuric acid, which is much stronger, more dangerous to handle, and more likely to damage your starting material. Secondly, this reaction can be carried out without solvent, as the reactants are miscible in each other. This greatly reduces waste. Mechanism for the dehydration of cyclohexanol H O OH H H OH2 H H2O : Safety precautions: Although green chemistry reduces safety precautions, it is difficult to eliminate them all together. Cyclohexene is easily flammable. Phosphoric acid is not strong like sulfuric acid, but skin contact should be avoided. Any spills should be cleaned immediately. This is our second synthesis, the procedure for synthesis prelabs will be followed again. You need a balanced reaction showing stereochemistry and a table of reactants, like the one below. Don’t forget to fill it out in your PreLab using the amounts in the instructions. Table of Reagents and Products name of b.p. m.w. mL density grams moles ratio of moles compound used (g/mL) used used theory used cyclohexanol 100.2 0.948 1 1 H3PO4 (85% w/w) cyclohexene - 98.0 - 82.1 0.811 - Limiting reagent _________________________________ Figure 1. Fractional distillation apparatus, column packed with copper Only the clamp holding the round bottom flask is tight. The remaining clamps are loose (support only). The (U-shaped) Clausen Adapter will be replaced with a 3-way connector today. Reaction Instructions: 1. Prepare a hot water bath in a 250 mL beaker on a hot plate/stirrer. 2. Place 0.075 moles cyclohexanol, 1.75 mL of 85% H3PO4, and a small magnetic stirrer in a 25 mL round bottom flask. 3. Set up the flask with a fractionating column, distillation head, thermometer, condenser, vacuum adaptor, and receiving flask. Connect a calcium chloride drying tube via rubber tubing to the vacuum adaptor. Only the bottom clamp should be tight. The rest should be loose, but supportive. 4. Heat at a gentle reflux for about five minutes, then heat the flask more vigorously to distill the mixture into the collection flask. Keep going until only 1 mL is left in the distillation flask, then remove the heat source. Record the boiling point. The water bath should get hot enough to distill your material. If not, switch to a sand bath. Isolation: 5. Place the distillate into a separatory funnel and wash with 5 mL distilled water. Carefully separate the layers and place the organic layer in a clean and dry Erlenmeyer flask. 6. Remove any visible water droplets before adding the sodium sulfate drying agent. Add a little of the drying agent, let stand for five minutes, swirling occasionally. A saturated drying agent will clump together. If the drying agent is in one big lump and no agent is loose, the sodium sulfate is saturated and more needs to be added and a further five minutes must be waited. When all water has been eliminated, the liquid will be clear and some sodium sulfate will not be clumpy. Characterization. 7. Determine the mass of your product and its refractive index and IR spectra. 8. Determine theoretical and percent yield. Post lab analysis: Analyze purity and yield of your product. In other words, what was your percent yield? Do you think that is good or bad? If your percent yield is poor, what do you think happened that reduced your yield? What do you think you could do next time to improve your yield? Did the color, refractive index, and bp of your product match the literature bp, color and refractive index? Was it even close? Is the IR give the expected peaks and no extra signals? Mark and identify key peaks on the IR. Post lab questions: 1. Complete the following reactions. Draw the most stable alkene formed without hydride or methyl shifts. You may use information on stability of alkenes found in chapter four. OH H+ heat a. OH H+ heat b. OH H+ heat c. 2. Draw mechanisms for the reactions in 1a and 1c, above, using H3O+ as your acid.