iiiii - ThinkChemistry
advertisement

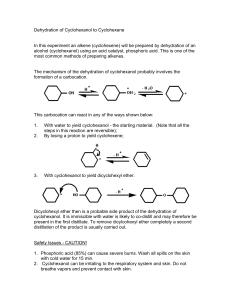
CHEMISTRY ORGANIC UNIT3 PPAs1-5 Thisisa dehydration reaction andnota condensation reaction because thewatermolecule hasbeenremoved from justonereactant molecule andnottwo. Toprepare yield. cyclohexene fromcyclohexanol anddetermine thepercentage Introduction Cyclohexene canbeprepared bydehydrating phosphoric usingconcentrated ryclohexanol acid.Thecyclohexene canbeseparated from thereaction mixture bydistillation and, afterpurification, it canbeweighed andthe percentage yieldcalculated. HH H\ Y ,H H-t-L-c/-oH | | H-!-.-c,-H H, A'H HH HH H \/ ,-\6-c-6-H ___> | ll H-!-..C-r., H, A HH +H,O Cyclohexanol is dehydrated toproduceEclohexene. Procedure About209of cyclohexanol to a pre-weighed wasadded round-bottomed flask,andtheflaskandcontents were reweighed accurately. Approximately 8 cm3of concentrated phosphoric acidwasaddeddropwise to thecyclohexanol. granuleS Afteradding a fewanti-bumping thereaction mixture wasslowlydistilled usingtheapparatus shown. reaction mixture anti-bumping granu res I I I I I t heat Whena reaction mixture is heated, thereis a tendency for it to boil violently aslargebubbles of superheated vapour suddenly eruptfromthe mixture. Thisis orevented bytheaddition of theantigranules. bumping Theliquidthatcameoverbetween 70oCand90oC was collected. Thedistillate waspouredintoa separating funnel andaboutanequalvolume of saturated sodium chloride solution wasadded. Theseparating funnelwas shaken thoroughly andleftto stand, allowing thetwo layers to separate. Theloweraqueous layerwasrunoff and - was disposed of,Theupperlayer- thecruderyclohexene runoffintoa smallconical flaskcontaining a fewpieces of anhydrous calcium chloridqa dryingagent. Thedried cyclohexene wasthendecanted intoa round-bottomed flasktogether witha fewanti-bumping granules.This crudecyclohexene wasslowlydistilled andtheliquidwhichcameoverbetween 80oCand85oCwascollected in a pre-weighed receiver flask.Theflaskwhichnowcontained purecyclohexene wasreweighed accurately, A fewdropsof thepurecyclohexene wereaddedto bromine solution to testfor unsaturation. Results = 30.46g Massof round-bottomed flask Massof round-bottomed flask+ cyclohexanol= 50.25g = 19'799 Massof ryclohexanol = 33'96g Massof receiver flask = 42.489 Massof receiver flask+ cyclohexene = 8.52g Massof cyclohexene From thebalanced eouation: In orderto calculate the 1 molcyclohexanol -+ 1 molcyclohexene percentage yield,youneed to knowtheaccurate mass 100.0g 82.0g (the of thelimiting reactant 1e.7eg <--------------- 1e'tex*#$ = $.23s onewhichis notin excess), andtheaccurate mass =ffix 100 = 52% %yield of thepureproduct that Thecyclohexene thatwasprepared in thisexperiment wasa colourless liquidwitha boiling isformed. pointof 80-85"C, lt turned theoranqe-red bromine solution colourless. 86 I