Carbonate or Bicarbonate?

Carbonate or Bicarbonate?
Name:_______________ Period:____
PURPOSE: To determine if an unknown compound is sodium carbonate or sodium bicarbonate by reaction with an acid and using basic mole calculations.
THEORY: As we have learned in class, the mole is the fundamental unit for the amount of a substance in chemistry. One mole of any element, which is 6.02 x 10 23 of that element, has as its mass the atomic mass in grams listed on the periodic table. Because the mole allows us to relate masses in grams to number of things, it allows us to calculate how many of each kind of atom are participating in a given chemical reaction.
To demonstrate the ideas behind mole calculations in the laboratory, we are going to do a simple experiment. Sodium carbonate and sodium bicarbonate are two ionic compounds that are frequently used in daily life. Sodium carbonate, Na
2
CO
3
, is also known as washing soda, and it is used a whitening agent and laundry booster for clothes washing and for making glass. Sodium bicarbonate, NaHCO
3
, is also known is baking soda and is used in baking and deodorizing. Both compounds react with an acid such as hydrochloric acid to form carbon dioxide, water, and sodium chloride.
Na
2
CO
3
NaHCO
(s) + 2HCl(aq) 2NaCl(aq) + CO
2
3
(aq) + HCl(aq) NaCl(aq) + CO
2
(g) + H
(g) + H
2
2
O(l)
O(l)
Because sodium carbonate contains two sodium cations, it will form twice as many moles of sodium chloride as sodium bicarbonate. You will use this difference to identify your unknown compound.
MATERIALS:
Evaporating dish
50, 100, or 150 ml beaker
Beral pipet
3 M HCl ( CAREFUL VERY CORROSIVE!)
Unknown salt sample
PRELAB: Please complete your standard prelab, including the tables below
Data Table I – Data on Unknown Salt Before and After Reaction with HCl
Letter of Unknown Salt:
Mass of Clean Evaporating Dish
Mass of Evaporating Dish and Salt
Mass of Weighing Boat
Mass of Weighing Boat + Salt
OBSERVATIONS:
CALCULATIONS: Be sure to show the following calculations clearly and completely in your lab notebook!
Part A – Carbonate or Bicarbonate.
1) Determine the mass of your salt by subtracting the mass of the salt and dish from the mass of the dish.
Mass of unknown salt:
2) You do not know if your unknown salt is sodium carbonate or sodium bicarbonate, but we can do some preliminary calculations that will help us in future steps. First, calculate the molar mass of sodium carbonate and sodium bicarbonate:
Molar mass of sodium carbonate:
Molar mass of sodium bicarbonate:
3) Now, assume your unknown was sodium carbonate. If this were true we can calculate how many grams of sodium chloride we would make. Calculate the number of grams of sodium chloride that could be made if your salt were sodium carbonate: ( write down on this page the example done in class so that you will know what you are doing when you write up your lab!)
Now do the same calculation, assuming your salt was sodium bicarbonate. Clearly show all of your steps.
4) Now we are ready to make some comparisons. Not e that the mass of the salt formed would be much less if your unknown were sodium bicarbonate. How much salt did you make? To answer this, calculate the mass of salt from your data table by subtracting the mass of the salt and the weighing boat from the mass of the salt.
Mass of Salt:
4) Based on your data and calculations, was your salt sodium carbonate or sodium bicarbonate? Explain.
DISCUSSION:
1) It is very likely that the amount of product you produced was less than the value you expected to obtain.
Why might that be? (you should be able to give at least two possible and legitimate reasons)
2) How could you prove, without tasting the compound, that the product you made was sodium chloride?
3) What do you think would have happened if we had used nitric acid in the reaction with your unknown instead of hydrochloric acid?
4) How many grams of product would have been made if your unknown had been 2.00 g of sodium phosphate?
Clearly show calculations that help you to determine this result
5) Write net ionic equations showing the reaction of HCl with aqueous sodium carbonate and aqueous sodium hydrogen carbonate
6) Calculate the percent composition of each element in sodium carbonate and sodium hydrogen carbonate.
BE SURE TO END YOUR LAB WITH A MEANINGFUL CONCLUSION!!!!!!
PROCEDURE: Carbonate or Bicarbonate
Day One
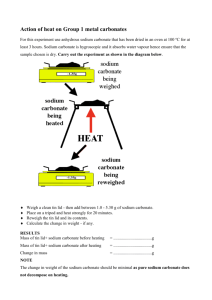
1) Form a lab group of 2 people and put on your apron and goggles!!!!
2) Obtain a clean evaporating dish and find its mass to 0.01 g. Write this value in your data table.
3) Place approximately 2.00 g of your unknown salt in your evaporating dish. Record the weight of dish and salt to the nearest 0.01 g. Note you do not have to have exactly 2.00 g, but keep the range between
1.80 to 2.25 g. If you weigh out too much salt, dispose of the excess by placing it in the sink and rinsing with water. NEVER RETURN SUBSTANCES THAT HAVE BEEN REMOVED TO THEIR
ORIGINAL BOTTLE!!!
To do so would likely contaminate the whole bottle.
4) Using the plastic pipet, slowly drop in 3M HCl into the dish, stirring occasionally with the stir bar.
Continue to add HCl until the solid has dissolved and no further bubbling occurs. Be careful not to add too much excess HCl! Record any observations.
5) Your instructor has set up a steam bath for you. Place the evaporating dish on top of the bath to help evaporate excess liquid.
6) Your solution will slowly boil off. When it looks mushy, ask the instructor to check your evaporating dish and steam bath. If it is ready, the instructor will remove the beaker and place the evaporating dish on the ring stand. GENTLY heat the dish with the flame from the Bunsen burner. If you do not heat gently the salt will splatter and you will lose product.
7) After the liquid has evaporated, turn off the burner flame. Place the name of your and your partner under the burner. The instructor will collect your dish and place it in the oven for you to complete drying.
Day Two:
1) Find the mass of an empty weighing boat
2) Obtain your evaporating dish and, using the rubber policeman, transfer all of your product from the dish to the boat.
3) Find the mass of the boat and product. Throw away the boat and rinse out your evaporating dish.