titration lab
advertisement

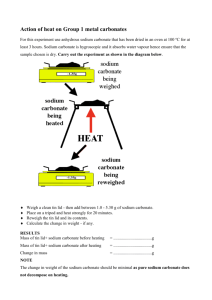
Date: Name: Partners: Titration Analysis of Hydrochloric Acid Problem: What is the molar concentration of a given hydrochloric acid solution? Design: A 0.100 mol/L standard solution of sodium carbonate is prepared. Measured samples of this solution are titrated with a sample of hydrochloric acid solution. The color change of methyl orange is the endpoint of the titrations. The titration is repeated until three consistent results are obtained, that is, until reacting volumes agree within 0.1 mL to 0.2 mL. Procedure: Prelab: Calculate the mass of sodium carbonate required to prepare 100.0 mL of a 0.100 mol/L solution. 1. 2. 3. 4. 5. 6. 7. 8. Prepare the standard solution of sodium carbonate and transfer it to a clean, dry, labeled 250 mL beaker. Place 70 – 80 mL of HCl in a clean dry labeled 100 mL beaker. Pour the HCl into the buret in its stand Pipette a 10.00 mL sample of the sodium carbonate solution into a clean Erlenmeyer flask and add 1 to 3 drops of methyl orange indicator. Record the initial buret reading to 0.1 mL Titrate the sodium carbonate solution with acid until a single drop produces a permanent change from pale yellow to pink. Swirl the flask during titration Record the final buret reading to 0.1 mL Repeat steps 5 to 8 until three consistent results are obtained. Observations: Trial 1 2 3 Colour Final reading (mL) Initial reading (mL Volume of HCl (mL) Analysis: 1. Balance the equation and do the stoichiometric calculation to find the wanted. (remember to use the volume found for Sodium carbonate – which is the purpose of doing a titration) 2. Calculate the % yield and % error 3. What is the purpose of the indicator? Explain. 4. Jeff used baking soda to neutralize a spill of battery acid (sulfuric acid) on his garage floor. What mass of baking soda would be required to completely neutralize 175 mL of 2.5 mol/L sulfuric acid? (8 marks)