Structured_questions
advertisement
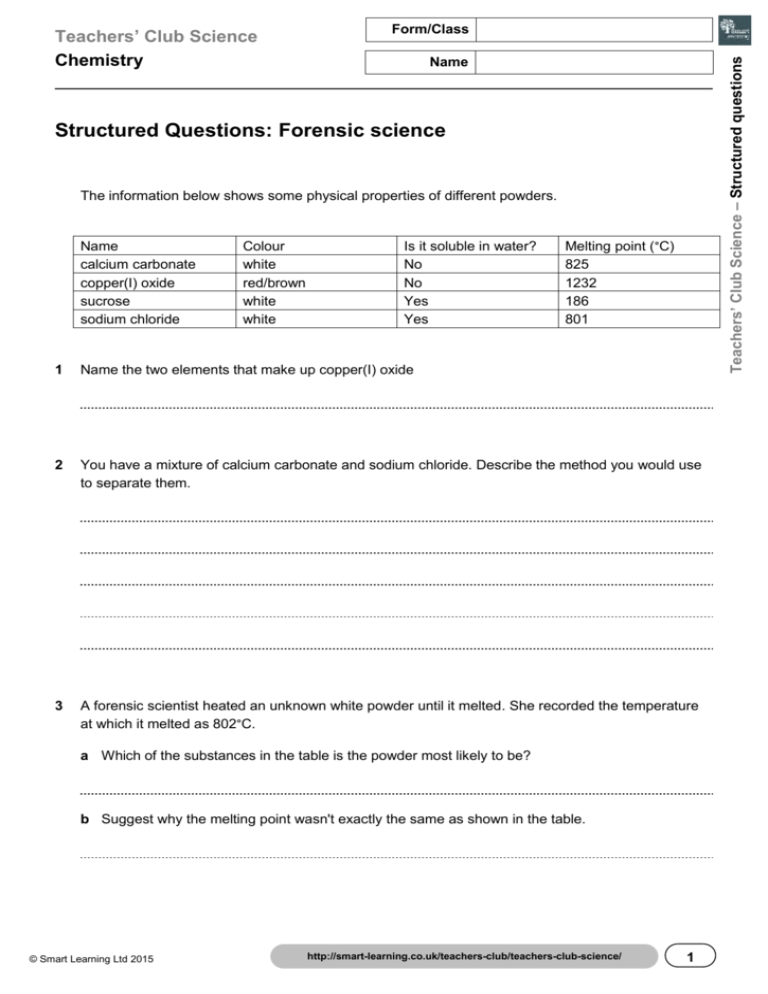
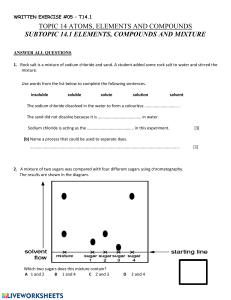
Form/Class Teachers’ Club Science – Structured questions Teachers’ Club Science Chemistry Name Structured Questions: Forensic science The information below shows some physical properties of different powders. Name calcium carbonate copper(I) oxide sucrose sodium chloride Colour white red/brown white white Is it soluble in water? No No Yes Yes Melting point (°C) 825 1232 186 801 1 Name the two elements that make up copper(I) oxide 2 You have a mixture of calcium carbonate and sodium chloride. Describe the method you would use to separate them. 3 A forensic scientist heated an unknown white powder until it melted. She recorded the temperature at which it melted as 802°C. a Which of the substances in the table is the powder most likely to be? b Suggest why the melting point wasn't exactly the same as shown in the table. © Smart Learning Ltd 2015 http://smart-learning.co.uk/teachers-club/teachers-club-science/ 1 Teachers’ Club Science – Structured questions Teachers’ Club Science Chemistry Form/Class Name Answers 1. Copper and oxygen 2. Add water to the mixture and stir. The sodium chloride will dissolve. Filter the mixture. The substance left on the filter paper is calcium carbonate. Leave the filtrate in an evaporating dish in a warm place. The water will evaporate leaving sodium chloride in the dish. 3. a. Sodium chloride b. It is contaminated with another substance, so it is not pure/it is a mixture. 2 http://smart-learning.co.uk/teachers-club/teachers-club-science/ © Smart Learning Ltd 2015