Section 8 Assignment
advertisement
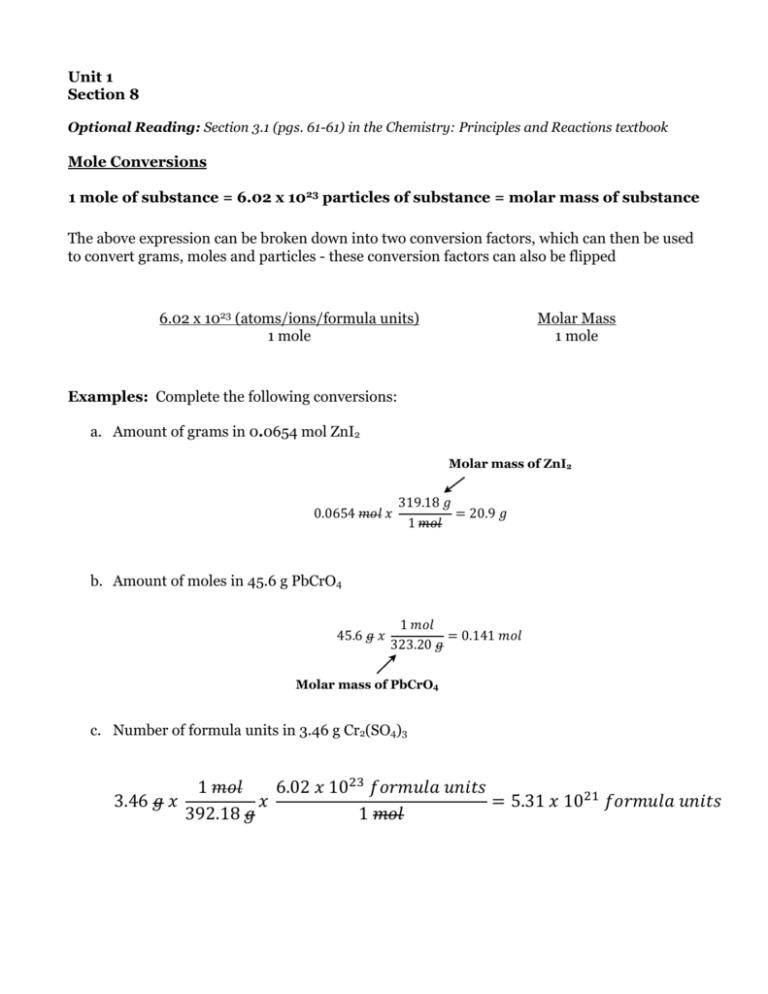
Unit 1 Section 8 !Optional Reading: Section 3.1 (pgs. 61-61) in the Chemistry: Principles and Reactions textbook ! Mole Conversions ! 1 mole of substance = 6.02 x 10 particles of substance = molar mass of substance ! 23 The above expression can be broken down into two conversion factors, which can then be used to convert grams, moles and particles - these conversion factors can also be flipped ! ! ! ! ! ! Molar Mass 1 mole 6.02 x 1023 (atoms/ions/formula units) 1 mole ! Examples: Complete the following conversions: ! a. Amount of grams in 0.0654 mol ZnI2 Molar mass of ZnI2 0.0654 𝑚𝑜𝑙 𝑥 319.18 𝑔 = 20.9 𝑔 1 𝑚𝑜𝑙 319.18 𝑔 0.0654 𝑚𝑜𝑙 𝑥 = 20.9 𝑔 1 𝑚𝑜𝑙 1 𝑚𝑜𝑙 b. Amount of moles in 45.6 g PbCrO 4 45.6 𝑔 𝑥 = 0.141 𝑚𝑜𝑙 319.18 𝑔 323.20 𝑔 0.0654 𝑚𝑜𝑙 𝑥 = 20.9 𝑔 1 𝑚𝑜𝑙 1 𝑚𝑜𝑙 45.6 𝑔 𝑥 = 0.141 𝑚𝑜𝑙 323.20 𝑔 1 𝑚𝑜𝑙 6.02 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 3.46 𝑔 𝑥 𝑥 = 5.31 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 392.18 𝑔 1 𝑚𝑜𝑙 Molar mass of PbCrO4 1 𝑚𝑜𝑙 1 𝑚𝑜𝑙45.6 𝑔 𝑥 6.02 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 = 0.141 𝑚𝑜𝑙 3.46 𝑔 𝑥 𝑥 = 5.31 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 323.20 𝑔 392.18 𝑔 1 𝑚𝑜𝑙 𝑎𝑡𝑜𝑚𝑠 c. Number of formula1 𝑚𝑜𝑙 𝑁𝐻 units in 3.463 𝑚𝑜𝑙 𝐻 g Cr2(SO4)6.02 𝑥 10 3 3.24 𝑔 𝑥 𝑥 𝑥 = 3.43 𝑥 10 𝑎𝑡𝑜𝑚𝑠 17.04 𝑔 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝐻 1 𝑚𝑜𝑙 𝑁𝐻 3 𝑚𝑜𝑙 𝐻 6.02 𝑥 10 𝑎𝑡𝑜𝑚𝑠 3.24 𝑔 𝑥 6.02 𝑥 10 𝑥 𝑥 = 3.43 𝑥 10 𝑎𝑡𝑜𝑚𝑠 1 𝑚𝑜𝑙 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 17.04 𝑔 (𝑃𝑂 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝐻 3.46 𝑔 𝑥 𝑥 1 𝑚𝑜𝑙 𝐶𝑎 = 5.31 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 ) 3 𝑚𝑜𝑙 𝐶𝑎 40.08 𝑔 4.71 𝑔 𝑥 𝑥 𝑥 = 1.83 𝑔 392.18 𝑔 1 𝑚𝑜𝑙 310.18 𝑔 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 1 𝑚𝑜𝑙 𝐶𝑎 ! 4.71 𝑔 𝑥 3.24 𝑔 𝑥 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 3 𝑚𝑜𝑙 𝐶𝑎 40.08 𝑔 𝑥 𝑥 = 1.83 𝑔 310.18 𝑔 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 1 𝑚𝑜𝑙 𝐶𝑎 1 𝑚𝑜𝑙 𝑁𝐻 3 𝑚𝑜𝑙 𝐻 6.02 𝑥 10 𝑎𝑡𝑜𝑚𝑠 𝑥 𝑥 = 3.43 𝑥 10 𝑎𝑡𝑜𝑚𝑠 17.04 𝑔 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝐻 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 3 𝑚𝑜𝑙 𝐶𝑎 40.08 𝑔 3.46 𝑔 𝑥 1 𝑚𝑜𝑙 𝑥 392.18 𝑔 1 𝑚𝑜𝑙 = 0.141 𝑚𝑜𝑙 6.02 𝑥 10 323.20 𝑔 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 45.6 𝑔 𝑥 1 𝑚𝑜𝑙 = 5.31 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 d. Number of hydrogen atoms in 3.24 g of NH3 1 𝑚𝑜𝑙 6.02 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 1 𝑚𝑜𝑙 𝑁𝐻 3 𝑚𝑜𝑙 𝐻 6.02 𝑥 10 𝑎𝑡𝑜𝑚𝑠 𝑥 = 5.31 𝑥 10 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑢𝑛𝑖𝑡𝑠 3.24 𝑔 𝑥 𝑥 𝑥 = 3.43 𝑥 10 𝑎𝑡𝑜𝑚𝑠 392.18 𝑔 1 𝑚𝑜𝑙 17.04 𝑔 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝐻 3.46 𝑔 𝑥 Subscripts represent the number of atoms of a particular element and/or the number of 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 3 𝑚𝑜𝑙 𝐶𝑎 moles)of that element in a formula 40.08 𝑔 1 𝑚𝑜𝑙 𝑁𝐻 3 𝑚𝑜𝑙 𝐻𝑥 6.02 𝑥 10 𝑎𝑡𝑜𝑚𝑠 𝑥 = 1.83 𝑔 3.24 𝑔 𝑥 4.71 𝑔 𝑥 𝑥 𝑥 = 3.43 𝑥 10 𝑎𝑡𝑜𝑚𝑠 310.18 𝑔 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 1 𝑚𝑜𝑙 𝐶𝑎 17.04 𝑔 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝐻 e. Amount of grams of Ca in 4.71 g of Ca3(PO4)2 4.71 𝑔 𝑥 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 3 𝑚𝑜𝑙 𝐶𝑎 40.08 𝑔 𝑥 𝑥 = 1.83 𝑔 310.18 𝑔 1 𝑚𝑜𝑙 𝐶𝑎 (𝑃𝑂 ) 1 𝑚𝑜𝑙 𝐶𝑎 ! Problems for Submission ! ! 1. Problem 1: How many carbon atoms are contained in 2.8 g of C2H4? 2. Problem 2: How many grams of nitrogen are in 25 g of (NH4)2SO4? 3. Problem 3: Determine the number of particles in each of the following: ! ! ! ! a. 62 g NH3 b. 14.9 g N2O5 c. 3.31 g NaClO4 4. Problem 4: Find the mass for the following mole amounts: ! a. 38 mol Na2SO3 b. 5.8 mol CO2 c. 48.1 mol K2CrO4 5. Problem 5: Find the amount of moles of substance in the following: ! a. 26.2 g Li2CO3 b. 41.4 g N2H4 c. 227 g Al2(SO4)3 ! !