Molar Mass & Mole Conversions: Chemistry Presentation
Learning Goals
0 Convert between grams and moles.
0 Find molar mass of an element.
The Mass of a Mole
0 Do 12 large nails have the same mass as 12 small nails?
The Mass of a Mole
0 Does 1 mol of sulfur have the same mass as 1 mol of carbon?
The Mass of a Mole
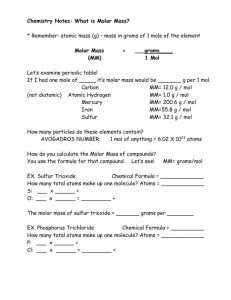
0 Molar mass is the mass in grams of one mole of any pure substance.
0 The molar mass of any element is numerically equivalent to its atomic mass and has the units g/mol.
The Mass of a Mole
= 55.845 g
Using Molar Mass
0 Converting from moles to mass:
Using Molar Mass
0 What is the mass of 3.00 moles of copper?
Using Molar Mass
0 Converting from mass to moles:
Practice
0 Determine the number of moles in each of the following:
0 25.5 g Ag
0 300.0 g S
Practice
0 Determine the mass of each of the following:
0 3.57 mol of Al
0 42.6 mol of Si
Using Molar Mass
0 How many atoms are in each of the following samples?
0 55.2 g Li
0 0.230 g Pb
0 11.5 g Hg
Using Molar Mass
0 What is the mass in grams of each of the following?
0 6.02 x 10 24 atoms Bi
0 1.00 x 10 24 atoms Mn
0 3.40 x 10 22 atoms He