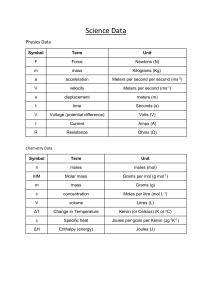
Mole Refresher Worksheet: Chemistry Conversions
advertisement
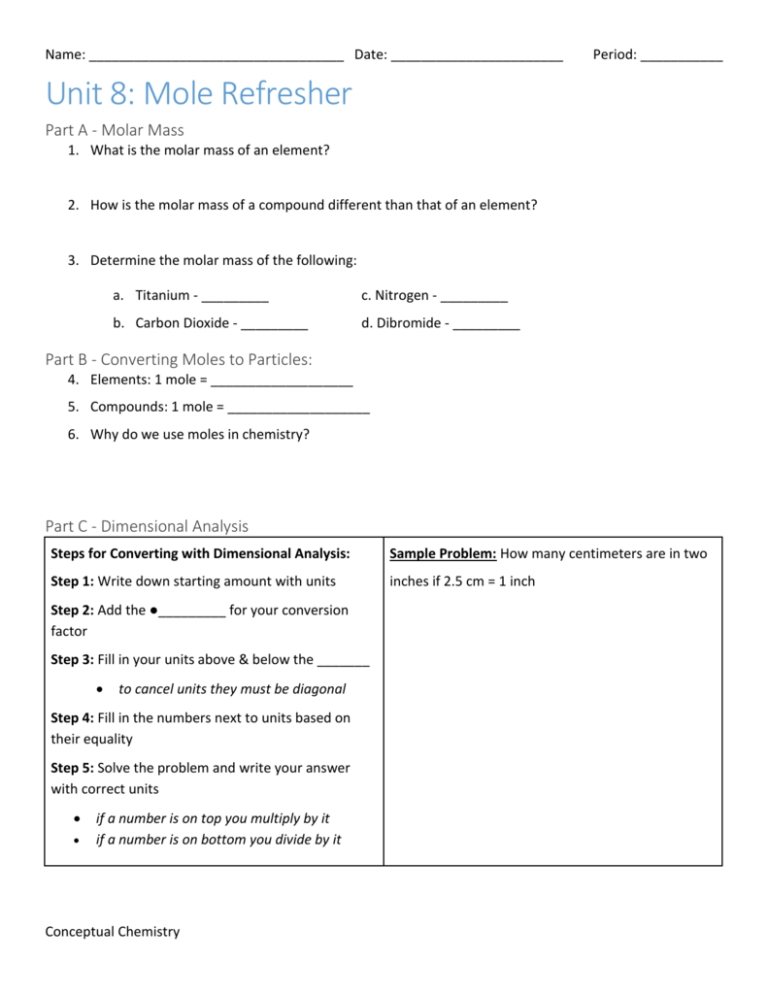
Name: __________________________________ Date: _______________________ Period: ___________ Unit 8: Mole Refresher Part A - Molar Mass 1. What is the molar mass of an element? 2. How is the molar mass of a compound different than that of an element? 3. Determine the molar mass of the following: a. Titanium - _________ c. Nitrogen - _________ b. Carbon Dioxide - _________ d. Dibromide - _________ Part B - Converting Moles to Particles: 4. Elements: 1 mole = ___________________ 5. Compounds: 1 mole = ___________________ 6. Why do we use moles in chemistry? Part C - Dimensional Analysis Steps for Converting with Dimensional Analysis: Sample Problem: How many centimeters are in two Step 1: Write down starting amount with units inches if 2.5 cm = 1 inch Step 2: Add the ●_________ for your conversion factor Step 3: Fill in your units above & below the _______ to cancel units they must be diagonal Step 4: Fill in the numbers next to units based on their equality Step 5: Solve the problem and write your answer with correct units if a number is on top you multiply by it if a number is on bottom you divide by it Conceptual Chemistry Name: __________________________________ Date: _______________________ Period: ___________ Conversions with Moles Using: Using: 7. You have 2.3 g of C. Convert to moles. 8. You have 5.1 mol of N. Convert to grams. 9. You have 7.022 x 1023 atoms of O. Convert to 10. You have 2.5 mol of Li. Convert to atoms. moles. 11. You have 1.21 x1024 molecules of H2O. Convert 12. You have 2.3 mol of H2. Convert to molecules. to moles. 13. You have 21.3 g of F2. Convert to moles. Conceptual Chemistry 14. You have 3.1 mol of HCl. Convert to grams.