Synthesis of 9
advertisement

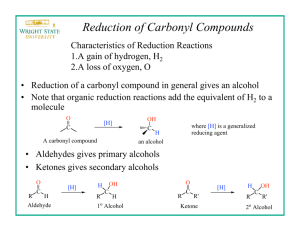
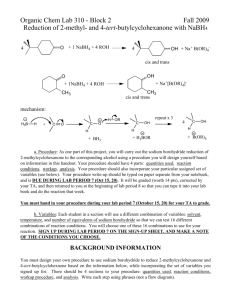
Zurich, 12.11.2007 Synthesis of 9-fluorenol OH Tobias Langenegger tobiasla@student.ethz.ch 05-918-362 D-Biol (chem.) assisted by Guo Xiaoqiang Method Reduction of a ketone to a alcohol using sodium borohydride as reactand. Chemical equation NaBH4 O OH MeOH Mechanism H H O B O H B H H H O O B O O H2SO4 OH H H Physical and safety data substance Mw (g/mol) density (g/cm3) mp (°C) bp (°C) Poison CH R-phrases S-phrases 9-fluorenone C13H8O 180.2 0.9 81-85 342 - 24, 25 - 9-fluorenol C13H10O 182.2 1.151 152-158 - - 24, 25 - MeOH CH3OH 32.04 0.787 -97.8 65 3 11, 23, 24, 25, 39 7, 16, 36, 37, 45 NaBH4 37.83 1.074 36 ~400 - 15, 25, 34 26, 36, 37, 38, 45 H2SO4 98.08 1.836 10.4 279.6 2 35 26, 30, 45 Equipment Vacuum filtration: reflux: Preparation Substance 9-fluorenone methanol NaBH4 H2SO4 eq 1 solvent 0.375 excess n (mmol) 3.385 147.4 1.269 37.43 V (mL) 6 2 m (mg) 610 48 - Experimental section 0.61 g 9-fluorenone was dissolved in 6 mL methanol under warming. After the solution was cooled down to RT, 48 mg NaBH4 was dissolved and let it react for 20 min. In that time the solution turned colorless. Than 2 ml of 3 M H2SO4 were added and the Solid product that accrued was heated with 2 pipettes of Methanol until it was completely dissolved. After cooling down to RT the flask was put in an ice-water bath for 15 min. Then the solid product was collected by vacuum filtration and washed with water. After that the 9-fluorenol was purified by a mixed solvent recrystallization using water and methanol in a ratio of 1 to 5.5. The Product was dried and characterized. Yield V - m 0.49 g n 2.69 mmol Yield 79.5% Characterisation Melting point Peaks in IR spectrum 153.7 – 154,2 °C ~3286 cm-1 ~3020 cm-1 1450 cm-1 (Literature 152 – 158 °C) O-H unsat. C-H aromatic ring Discussion The biggest problem was to dry the product. First it was dried for 20 min in a rotavap and for 30 min in the oven. But because it wasnʼt dry after this, it was putted in the oven for a day, the next week. In the end it was dry, what we can se on the IR. There isnʼt anymore this strong water peak, which was on the first IR. Literature https://www.discoverygate.com/ http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi http://www.chemexper.com/ http://www.wikipedia.org Attachment - Reference IR spectrum Copy of the lab notebook - IR spectrum Reference IR spectrum from http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi