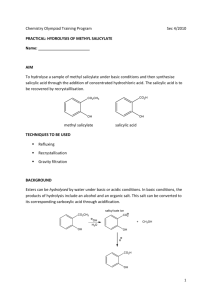
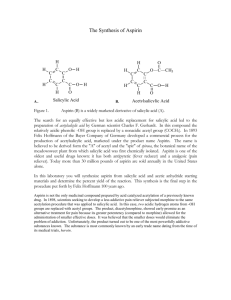
Synthesizing Aspirin and Oil of Wintergreen
advertisement
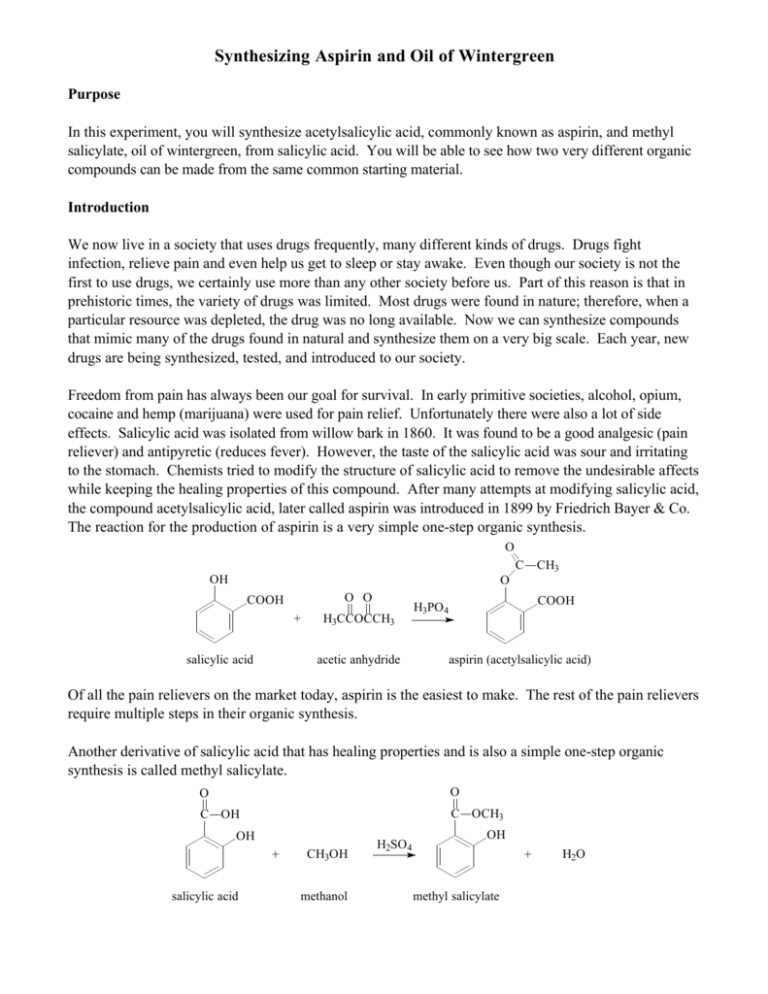
Synthesizing Aspirin and Oil of Wintergreen Purpose In this experiment, you will synthesize acetylsalicylic acid, commonly known as aspirin, and methyl salicylate, oil of wintergreen, from salicylic acid. You will be able to see how two very different organic compounds can be made from the same common starting material. Introduction We now live in a society that uses drugs frequently, many different kinds of drugs. Drugs fight infection, relieve pain and even help us get to sleep or stay awake. Even though our society is not the first to use drugs, we certainly use more than any other society before us. Part of this reason is that in prehistoric times, the variety of drugs was limited. Most drugs were found in nature; therefore, when a particular resource was depleted, the drug was no long available. Now we can synthesize compounds that mimic many of the drugs found in natural and synthesize them on a very big scale. Each year, new drugs are being synthesized, tested, and introduced to our society. Freedom from pain has always been our goal for survival. In early primitive societies, alcohol, opium, cocaine and hemp (marijuana) were used for pain relief. Unfortunately there were also a lot of side effects. Salicylic acid was isolated from willow bark in 1860. It was found to be a good analgesic (pain reliever) and antipyretic (reduces fever). However, the taste of the salicylic acid was sour and irritating to the stomach. Chemists tried to modify the structure of salicylic acid to remove the undesirable affects while keeping the healing properties of this compound. After many attempts at modifying salicylic acid, the compound acetylsalicylic acid, later called aspirin was introduced in 1899 by Friedrich Bayer & Co. The reaction for the production of aspirin is a very simple one-step organic synthesis. O C OH CH3 O O O COOH + salicylic acid H3CCOCCH3 acetic anhydride COOH H3PO4 aspirin (acetylsalicylic acid) Of all the pain relievers on the market today, aspirin is the easiest to make. The rest of the pain relievers require multiple steps in their organic synthesis. Another derivative of salicylic acid that has healing properties and is also a simple one-step organic synthesis is called methyl salicylate. O O C OH C OCH3 OH + salicylic acid CH3OH methanol H2SO4 OH + methyl salicylate H2O Methyl salicylate (oil of wintergreen) is used as a flavoring agent and it is also used in rubbing compounds, like Ben Gay. When methyl salicylate is applied to the skin, it causes a mild burning sensation which serves as a counterirritant for sore muscles. You will synthesize both of these compounds in this experiment and observe first hand how compounds can look so different physically but be quite similar chemically. In addition to the synthesis, you will analyze aspirin by a technique called infrared spectroscopy. Infrared (IR) spectroscopy uses infrared light to vibrate the bonds in an organic molecule. Chemical bonds in different environments will absorb varying intensities and at varying frequencies. Thus IR spectroscopy involves collecting absorption information and analyzing it in the form of a spectrum. The frequencies at which there are absorptions of IR radiation (peaks) can be correlated directly to bonds within the compound. Although IR can seem complicated, it can be simplified with a familiar analogy. Like a human fingerprint, each organic compound has a unique spectrum. The IR spectrum is essentially a chemical fingerprint for molecules. Using a chemical database, you’ll be able to confirm the identity of your newly synthesized drugs. Procedure Part I: Synthesis of Aspirin 1. Heat approximately 150 mL of water in a 250 mL beaker using a hot plate. (Set-up the hot plate under the hood.) 2. To a large test tube, add 1 g of salicylic acid, a boiling chip, and 8 small drops of 85% phosphoric acid (H3PO4.) 3. Add 2 mL of acetic anhydride. Use the acetic anhydride to wash the other reagents to the bottom of the test tub. 4. Mix the reactants thoroughly with a glass stirring rod, and then heat the reaction tube in the beaker of hot water at ~90°C for 5 min. 5. Cautiously add 1.5 mL of d H2O dropwise to the reaction mixture. This step will decompose excess acetic anhydride and be exothermic (give off heat.) 6. When the reaction is over, add 2 mL more water and allow the tube to cool slowly to room temperature. Allow the solution to sit for 10 min. If crystallization does not occur during the cooling process, add a seed crystal or scratch the inside of the tube with a glass stirring rod. 7. Cool the tube in ice until crystallization is complete, and then remove the crystals using vacuum filtration. Wash the crystals with a very small quantity of ice cold water. 8. Place the product onto a piece of filter paper and squeeze the crystals between sheets of filter paper to absorb excess water. Allow to air dry while performing Part II of this experiment. 9. After you have completed Part II, weigh the crystals and record the weight. 10. With the help of the instructor, prepare and obtain an IR spectrum of your product. Part II: Synthesis of Wintergreen 1. Weigh approximately 1g of salicylic acid and place in a test tube. 2. Add 10mL of methanol to the test tube and gently shake the test tube until all of the salicylic acid has dissolved. 3. Carefully add 20 drops of concentrated sulfuric acid (H2SO4) to the test tube. 4. Stir the contents in the test tube with a stirring rod. 5. Place the test tube in the water bath for approximately two minutes. 6. Remove the test tube from the water bath and carefully sniff the contents. If you smell wintergreen then you have made methyl salicylate. Results Mass of aspirin produced _________ Identification of aspirin (rate: Excellent, good, fair, or poor match) _________ Print out IR spectrum and attach to report Questions: 1. Describe the smell of methyl salicylate. 2. Provide two practical applications for IR.