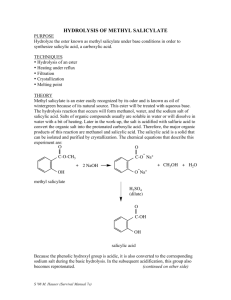
Hydrolysis of Methyl Salicylate
advertisement

Chemistry Olympiad Training Program Sec 4/2010 PRACTICAL: HYDROLYSIS OF METHYL SALICYLATE Name: AIM To hydrolyse a sample of methyl salicylate under basic conditions and then synthesise salicylic acid through the addition of concentrated hydrochloric acid. The salicylic acid is to be recovered by recrystalllisation. methyl salicylate salicylic acid TECHNIQUES TO BE USED Refluxing Recrystallisation Gravity filtration BACKGROUND Esters can be hydrolysed by water under basic or acidic conditions. In basic conditions, the products of hydrolysis include an alcohol and an organic salt. This salt can be converted to its corresponding carboxylic acid through acidification. 1 SAFETY CONSIDERATIONS Care should be taken when handling concentrated hydrochloric acid as it is very corrosive. Please call your tutor or lab demonstrator before handling the acid. It should also remain in the fumehood. Appropriate care should be ensured whenever operating a heating implement such as the hot plate. Handle all hot glassware carefully by using the oven mitts provided. PROCEDURE Reflux of reaction mixture (In groups of four) 1. 2. 3. 4. Add 3.0 cm3 of methyl salicylate into the 250 cm3 round-bottom flask. Measure 30 cm3 of 2 mol dm-3 sodium hydroxide and add it to the same round-bottom flask. Reflux the mixture until a clear homogeneous solution is obtained. Allow the solution to cool before transferring the contents into two conical flasks in equal proportion, one for each pair in the group. Recrystallization of salicylic acid (In pairs) 5. 6. 7. 8. Add concentrated hydrochloric acid to the solution until it is completely acidified. Boil the resulting mixture, adding distilled water until all the precipitate dissolves to give a clear homogeneous solution. Allow the solution to cool and crystallize the salicylic acid. Separate the crystals from the solution by suction filtration. 2 Results, Observations and Calculations Record all your observations throughout the progress of your experiment. Given the following information, calculate the percentage yield of salicylic acid obtained in the experiment. Mass of pure salicylic acid collected = 2.74 g Density of methyl salicylate = 1.17 g dm-3 Mr of methyl salicylate = 152.1 g mol-1 3