File - Synthesizing Methyl Salicylate
advertisement

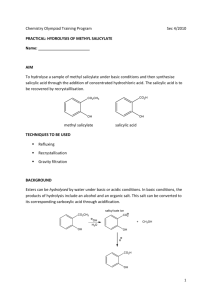
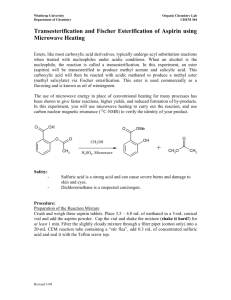
Introduction Many over the counter products carry a strong and pleasant mint odor that originates from the wintergreen-scented chemical, have you ever wondered how this smell forms? That smell can be attributed to a funky molecule called oil of wintergreen. Oil of wintergreen, or methyl salicylate, is actually an organic type of ester, which means that it is a combination of alcohol with an organic acid (methanol and aspirin). An ester is any class of compounds produced by the reaction between acids and alcohols with the elimination of water. Wintergreen oil is used typically as aroma therapeutic folk remedy for muscle and joint discomfort, flavoring agent for toothpaste, chewing gum, soft drinks, confectionary, and in mint flavorings. This flavor can be produced by scientists in a laboratory or factory through artificial means. Many factories and companies prefer using this method of obtaining the oil of wintergreen for the reason that it is much more affordable than actually growing the plants needed to acquire the minty smell that the oil produces. However, many studies have proven that oil of wintergreen can be dangerous in high concentrations as well as having many fatal consequences. A comprehensive review of the existing medical literature on methyl salicylate poisoning was performed, and data compiled over the past two decades by the American Association of Poison Control Centers (AAPCC) was examined. Proved that Methyl salicylate continues to be a relatively common source of pediatric exposures as a result persistent reports of life-threatening and fatal toxicity were found. In children less than 6 years of age, a teaspoon (5 mL) or less of oil of wintergreen has been implicated in several well-documented deaths. In adults, Wintergreen taken in levels above 5 mL can cause severe vomiting and death, with the danger increasing as the amount increases. Although, methyl salicylate can be harmful other research says otherwise. Wintergreen essential oil is also well known to eradicate pain and drive out stress and tension. The relaxing and stimulating effect of this oil reduces spasms in the respiratory, muscular, digestive and nervous systems. Methyl salicylate is also known to induce numbness when rubbed on affected area. This can also increase circulation of blood and furthermore “fight” the pain. The high degree of toxicity of this oil can make it fatal to human beings, but that means that it is fatal to bacteria and other microbes such as viruses, fungi and protozoa as well. This quality is utilized to fight infection that causes sepsis. As a result scientist conduct plenty of research before performing an experiment to determine the pleasing and awful factors of any chemical. They additionally calculate many factors that will be needed for the experiment. Often times, scientist calculate the density of their solutions. All objects have density which can increase or decrease as the result of the actions being taken on the object. Density changes with increasing mass or with decreasing volume. Therefore, you can alter the density of an object by increasing the mass or by decreasing the volume. To determine the density of any given substance, scientist must determine the mass and volume. To determine the mass, requires the mass of the solution and beaker subtracted from the mass of the blank beaker. Mass of Methyl Salicylate = Mass of Beaker and Solution – Mass of Empty Beaker After determining mass and volume density is calculated by mass of substance over volume of substance. Density = Mass of substance Volume of substance In chemistry, it is sometimes important to know how efficient a reaction is. Many times, the final product of an experiment can be completely different from what was originally predicted. This is where finding the percent yield becomes greatly vital. Percent Yield can determine many factors for example the precision of a reaction and how precise the reaction genuinely was. Percent yield can be solved by dividing was initially predicted to compose from what was actually composed. Throughout the year many intriguing topics have been covered and discussed. In the lab of synthesizing Methyl salicylate, the main topics that were covered through the process were: the freezing point of a pure substance, Triple point in which all phases of the product existed and Critical point were two phases of the substance become indistinguishable from each other. These topics which were discussed are important in order to understand the complexity of Phase diagrams. Heating curves were also an essential factor seen in the process of synthesizing methyl salicylate. When heating the substance to obtain the wintergreen smell the relationship of temperature and time formed vapor which then condensed. Although these topics are not commonly noticed, the process of vapor condensing is actually seen in unnoticeable processes like heating a substance which happens in daily lives. It is known that many companies and labs use the process of synthesizing menthyl salicylate as result it is much cheaper to produce. An important factor taken into consideration within the actual lab was to interpret an actual procedure lab technicians perform in similar labs, to obtain the oil of wintergreen, in a much comprehensible way using common household items. The purpose of this lab was to imitate this procedure in order to understand why it is more convenient to use these substances as well as to determine how close the theoretical yield can get to the actual yield. The information researched helped the process of the lab, considering that it made the understanding much easier as well helped carry out the procedure out which then later contributed to the comprehension of what actually was expected of the lab. Background information was a useful resource in providing enough knowledge to form a hypothesis. In addition the final goal of the process was to apprehend why methyl salicylate, a main ingredient in common foods, is dangerous in its natural state but safe to consume when occupied with other ingredients. As many other great scientists who perform labs and create a hypothesis this routine was also mocked in this procedure. The hypothesis for this lab prior to background research came about to be: If methyl salicylate is added to other chemicals and further broken down, Then it would become harmless to consume in common food because it become less harmful once it turns into a new compound. This hypothesis helped determine how accurate our understanding of the lab was prior to our research along with our pre-lab questions.