Chapter 5 and 6
advertisement
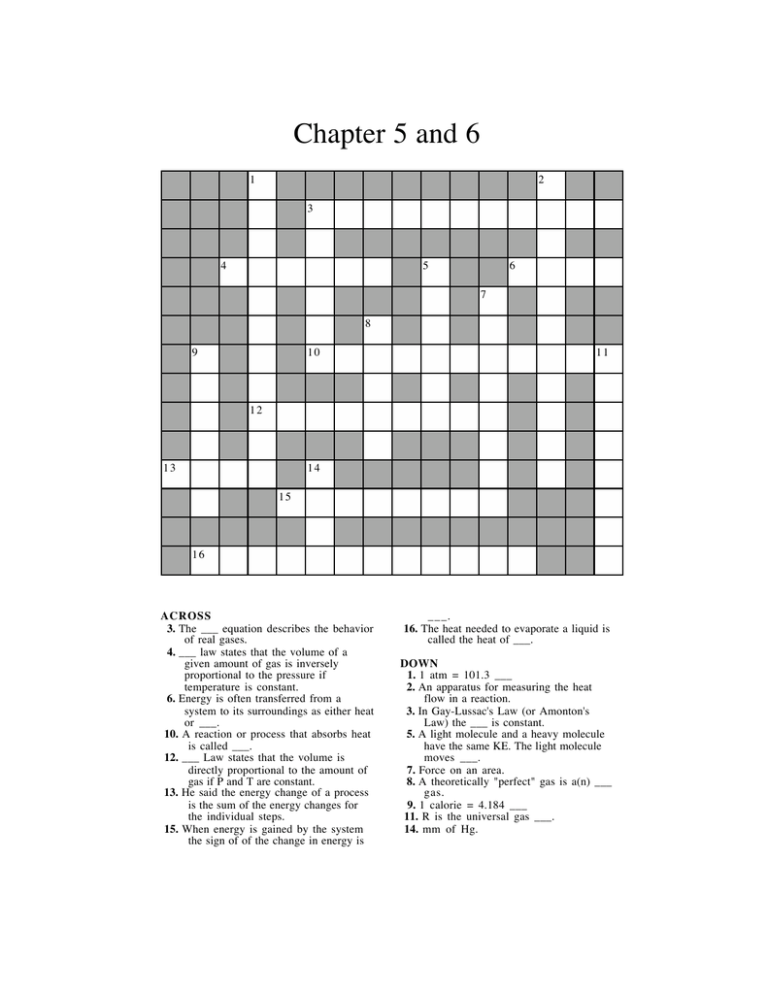
Chapter 5 and 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 ACROSS 3. The ___ equation describes the behavior of real gases. 4. ___ law states that the volume of a given amount of gas is inversely proportional to the pressure if temperature is constant. 6. Energy is often transferred from a system to its surroundings as either heat or ___. 10. A reaction or process that absorbs heat is called ___. 12. ___ Law states that the volume is directly proportional to the amount of gas if P and T are constant. 13. He said the energy change of a process is the sum of the energy changes for the individual steps. 15. When energy is gained by the system the sign of of the change in energy is ___. 16. The heat needed to evaporate a liquid is called the heat of ___. DOWN 1. 1 atm = 101.3 ___ 2. An apparatus for measuring the heat flow in a reaction. 3. In Gay-Lussac's Law (or Amonton's Law) the ___ is constant. 5. A light molecule and a heavy molecule have the same KE. The light molecule moves ___. 7. Force on an area. 8. A theoretically "perfect" gas is a(n) ___ gas. 9. 1 calorie = 4.184 ___ 11. R is the universal gas ___. 14. mm of Hg.