Vapor Pressure and Evaporative Cooling Lab
advertisement

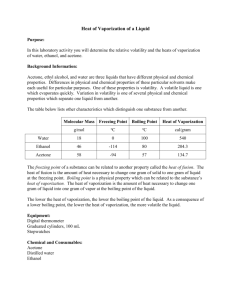
Vapor Pressure and Evaporative Cooling Name: _____________________________________________ Date: _______________ PROBLEM: How does vapor pressure effect evaporation? MATERIALS: 1 piece each of filter paper soaked in propanone (acetone); water; and ethanol; 1 thermometer PROCEDURE: 1. Record the initial temperature of the thermometer in the air in the data table below. 2. Wrap the bulb of the thermometer with one of the soaked pieces of filter paper. Make sure that your hand is not touching the bulb of the thermometer. 3. Watch the temperature for several minutes. As the liquid evaporates, the temperature will begin to drop. 4. Make note of the lowest temperature each thermometer reaches, and record it in the data table below. 5. Repeat steps 1 through 4 for the other two pieces of liquid-soaked filter paper. 6. Calculate the absolute value of the temperature change for each liquid by subtracting the coldest temperature reached by each from its initial temperature. 7. Determine the normal boiling point of each liquid by looking at Table H, “Vapor Pressure of Four Liquids”, on your reference tables. Record the boiling points in the data table below. 8. On a separate sheet of graph paper, prepare a graph of normal boiling point versus temperature change. Use the x-axis for boiling point and the y-axis for temperature change. Plot the points and draw the best straight line or curve through the points. OBSERVATIONS: Liquid Initial Temperature Lowest Temperature Reached Absolute Temperature Change Normal Boiling Point CONCLUSIONS: 1. Why does the temperature drop when the liquid evaporates from the wet paper? 2. What is the relationship between the normal boiling point of a liquid and how well it cools by evaporation? 3. Use your graph to predict the lowest temperature a thermometer wet with isopropyl alcohol would reach if its boiling point is 82.4°C. 4. Draw the relative number of vapor particles for each of the three liquids at the same temperature in the containers below. What is the relationship between the vapor pressure of a liquid and how well it cools by evaporation? Water Ethanol Acetone 5. Which of the liquids you tested has the strongest forces holding its molecules to each other? What evidence does the laboratory investigation provide to support your answer?