Heat of Vaporization of a Liquid
advertisement
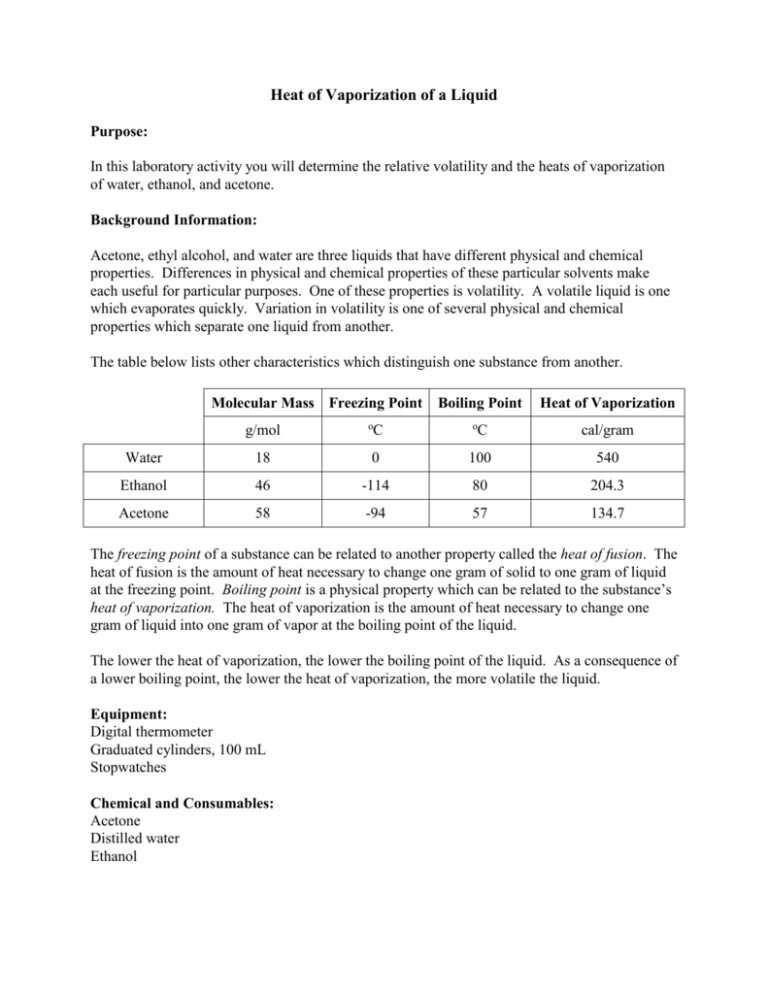
Heat of Vaporization of a Liquid Purpose: In this laboratory activity you will determine the relative volatility and the heats of vaporization of water, ethanol, and acetone. Background Information: Acetone, ethyl alcohol, and water are three liquids that have different physical and chemical properties. Differences in physical and chemical properties of these particular solvents make each useful for particular purposes. One of these properties is volatility. A volatile liquid is one which evaporates quickly. Variation in volatility is one of several physical and chemical properties which separate one liquid from another. The table below lists other characteristics which distinguish one substance from another. Molecular Mass Freezing Point g/mol o C Boiling Point o Heat of Vaporization C cal/gram Water 18 0 100 540 Ethanol 46 -114 80 204.3 Acetone 58 -94 57 134.7 The freezing point of a substance can be related to another property called the heat of fusion. The heat of fusion is the amount of heat necessary to change one gram of solid to one gram of liquid at the freezing point. Boiling point is a physical property which can be related to the substance’s heat of vaporization. The heat of vaporization is the amount of heat necessary to change one gram of liquid into one gram of vapor at the boiling point of the liquid. The lower the heat of vaporization, the lower the boiling point of the liquid. As a consequence of a lower boiling point, the lower the heat of vaporization, the more volatile the liquid. Equipment: Digital thermometer Graduated cylinders, 100 mL Stopwatches Chemical and Consumables: Acetone Distilled water Ethanol Procedure: 1. Obtain a graduated cylinder containing one of the three liquids to be tested. 2. Place the digital thermometer into the cylinder and leave it in the liquid at least 30 seconds. Note and record the temperature of the liquid. 3. Take the thermometer out of the liquid and observe the temperature at all times. The partner should begin timing the process. Hold the thermometer vertically and record the highest temperature. 4. Continue observing the temperature as the thermometer cools until the temperature begins to level. Stop timing and record the lowest temperature observed. 5. Rinse off the thermometer with water and carefully dry it with a paper towel. 6. Repeat the above procedure with each of the other two liquids.