IMFAs - iteach.org
advertisement

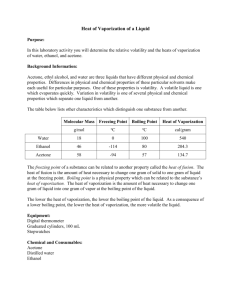
IMFA Lab Background: Many properties of chemicals are determined by the strength of the forces holding the molecules together. For example, gases like Nitrogen (N2) and Helium (He) have very weak attractive forces between individual molecules – which is why they are gases. Liquids have stronger attractive forces than gases, and solids have even stronger forces. By determining the differences in boiling points and the time it takes to evaporate, we can determine the relative strengths of these forces within similar compounds! Materials: Hot Plate Ethanol Droppers Distilled Water Thermometer Timer or clock Acetone Erlenmeyer Flasks Ring Stand w/ Thermometer clamp Objective: Determine the relative strengths of the IMFAs of three clear liquids. Procedure: I. Evaporation Rate 1. Have someone watch the clock or use a timer 2. Place three drops of each liquid on the lab table 3. Record the time it takes for each liquid to evaporate completely. II. Boiling Point 1. Place about 50 mL of distilled water into a small Erlenmeyer flask. 2. Place the flask on the hot plate and carefully heat. 3. Lower the clean thermometer into the liquid (not touching the bottom of the beaker) 4. Record the temperature when the liquid boils. 5. Repeat with Acetone and Ethanol. Observations: LDS: Draw the Lewis Dot Structures for each compound. If you have problems with them, see your teacher! Compound Formula Water H2O Acetone CH3COCH3 Ethanol CH3CH2OH LDS Questions: 1. Put the three compounds in order from the one with the highest intermolecular forces of attraction (IMFAs) to the one with the lowest. 2. What role does molecular weight have to do with the strengths of IMFAs? 3. What role does bond type have to do with the strengths of IMFAs? 4. Which would you predict to have a higher boiling point, CH2O or CH3OH? Why?