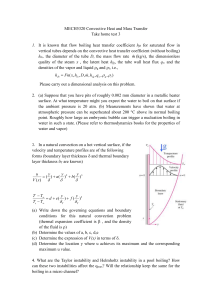
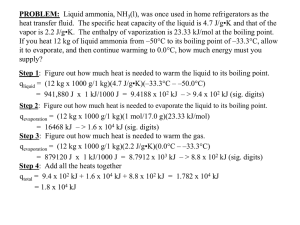
Difference between Boiling & Evaporation
advertisement

www.SimpleChemConcepts.com Difference between Boiling & Evaporation: 1 2 3 Boiling Happens at Boiling Point of liquid Occurs throughout the liquid Faster Evaporation Happens below Boiling Point of liquid Occurs only at the surface of the liquid Slower Extra Notes: Temperature at which this happens is known as Boiling Point Liquids with boiling points that are not very far above room temperature evaporate very easily, and are called Volatile Liquids Action: “People may doubt what you say, but they will believe what you do” - Lewis Cass Copyright 2008 @ SimpleChemConcepts.com