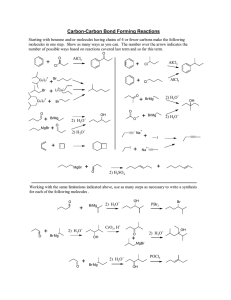
Key to In-Class Group Activity 8 Chem 102, Fall 2005 (Not Graded)

Key to In-Class Group Activity 8
Chem 102, Fall 2005
(Not Graded)
1. Give the major product for each of the following reactions (use NR for no reaction):
OH
H
3
O
+ a) heat
CH
3
CH
3 b) 2
OH
H
3
O
+ heat
O c) 2
SH
I
2
S
S d) e) f) g)
OH
CH
3
PCC
OH
PCC
OH
CrO
3
H
3
O
+
OH CrO
3
H
3
O
+
O
H
O
CH
3
O
OH
NR
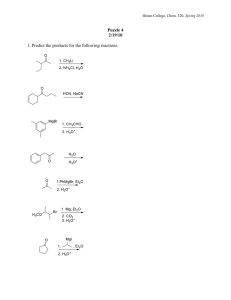
2. Give the reactants (and any catalysts) that would form each of the following products:
OH PCC O a) or CrO
3
/H
3
O
+ b) 2 CH
3
SH
I
2
S
S c) d)
OH
OH
CrO
3
H
3
O
+
H
3
O
+ heat
O
OH e)
OH
PCC
O
H
2 CH
3
OH
H
3
O
+
O f) heat
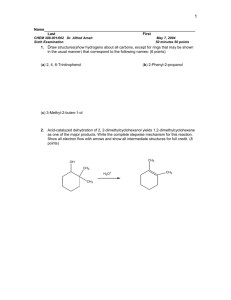
3. Write the arrow-pushing mechanism for the reaction in question 1(b) above:
H H
O
+
O
+
OH O H H
H H H
OH
+
O
H
H
O
H
+
H
O
H
O
H
+
H
O
H
O
+
H
H
O
H
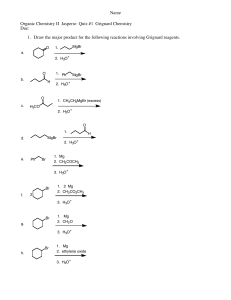
4. Write the arrow-pushing mechanism for the following reaction:
OH H
3
O
+ heat
OH
+
H
H
O
H
H
O
H
+
H
O
H
H
O
H
+
H
O
H
H
+
O
+
O
H H H
H H
5. Why is it that when you heat a secondary or tertiary alcohol with aqueous acid the major product is an alkene (elimination reaction), while when you do the same thing with a primary alcohol you get an ether (substitution reaction)? Use drawings
(chemical structures) to illustrate your answer.
The secondary and tertiary alcohols can form more stable carbocation intermediates than primary alcohols. The primary alcohols can’t form a stable carbocation intermediate, so they undergo substitution reactions.
OH
More Substituted
OH
Less Substituted