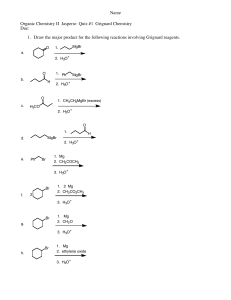
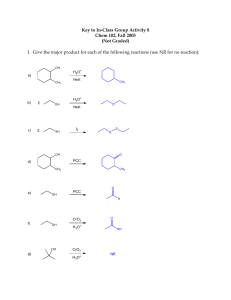
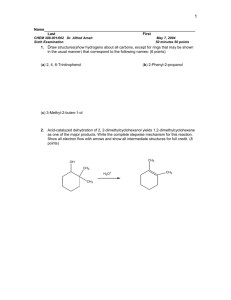
Predict signals in 13C NMR
advertisement
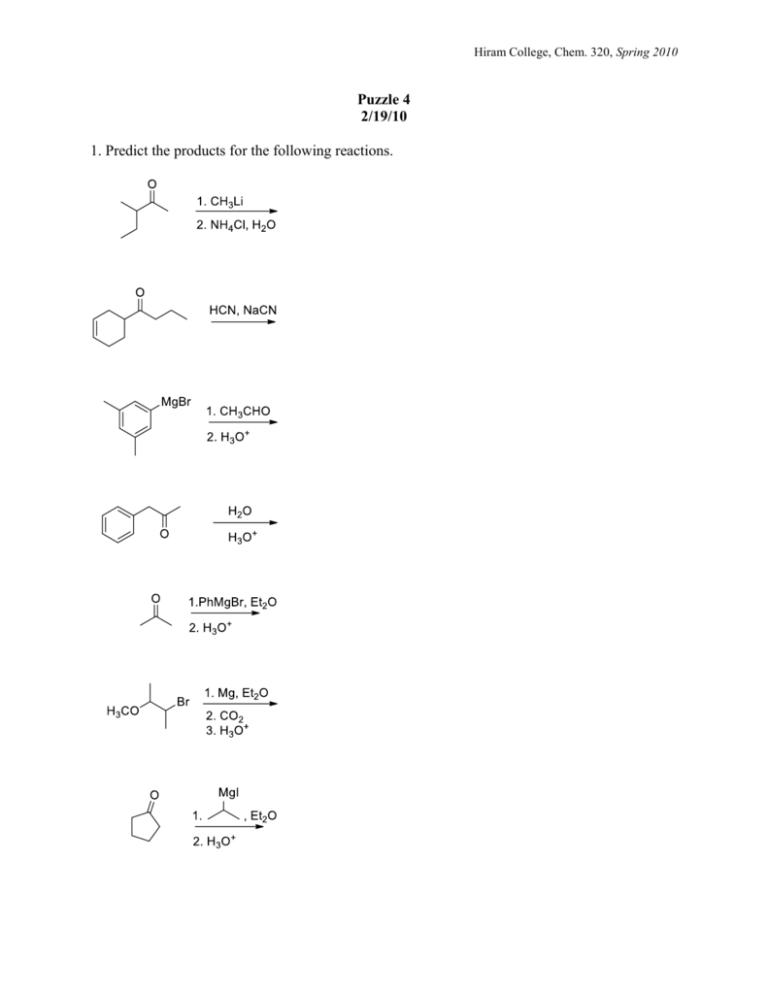
Hiram College, Chem. 320, Spring 2010 Puzzle 4 2/19/10 1. Predict the products for the following reactions. Hiram College, Chem. 320, Spring 2010 2. Do you remember these reactions? Show syntheses of these compounds. You may start with any alcohols containing four or less carbons, any inorganic reagents, and any solvents you need. 3. Many of the nucleophilic addition reactions to carbonyl carbons are actually equilibrium processes. The addition of a Grignard, a hydride, or an acetylide is not a equilibrium process. Why? Hiram College, Chem. 320, Spring 2010 4. Addition of alcohols to aldehydes and ketones produces acetals and ketals, respectively. One example of such a reaction is shown below. Propose a reasonable mechanism for the following transformation. 5. Using the information above as a guide, predict the products of the following reactions. Hiram College, Chem. 320, Spring 2010 6. Nitriles are good electrophiles, and tend to undergo reactions similar to those of carbonyl compounds. One nucleophile they react with quite well is a Grignard reagent. After an acidic work-up, the product of this sequence is a ketone. Propose a reasonable mechanism for the following transformation. 7. Based on what you reasoned above, predict the products for the following reactions. CH3CH2CH2 C N: 1) EtMgBr 2) H3O+ 1) PhMgBr 2) H3O+ (CH3) 2CHCH2 C N: CH3 1) MeMgBr 2) H3O+ C N: