Atomic Mass of Noodlium
advertisement

Name: _________________________
Date: _________________
Atomic Mass of Noodlium
Materials and Equipment
• Triple beam balance
• A Sample of Noodlium
Procedure
1. Obtain a sample of “noodlium” from your teacher. It contains a mixture of different isotopes,
i.e. pasta noodle varieties.
2. Measure and record the total mass of the sample in the baggie
_________________ g
total mass of sample
3. Carefully empty the contents onto a large flat surface. Measure the mass of the empty baggie:
_________________ g
mass of the baggie
What is the mass of the sample alone?
_________________ g
sample mass
4. Sort and record the “pasta noodles” by type. Count the total number of noodles.
5. Find the average mass of each type of “pasta noodle”. Determine the average by measuring the
mass of 10 noodles of each type and dividing by 10.
Type of noodle
Average mass (g)
(a) _________________
_________________
(b) _________________
_________________
(c) _________________
_________________
(d) _________________
_________________
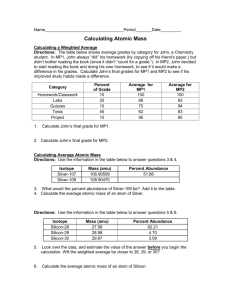
6. Calculate the percentage of each noodle type (isotope) in the sample, using the formula:
% abundance = # of noodles of a given type x 100%
Total Number of Noodles
Name: _________________________
Date: _________________
Type of Noodle
% abundance
(a) _________________
_________________
(b) _________________
_________________
(c) _________________
_________________
(d) _________________
_________________
7. Determine the weighted average atomic mass for noodlium using the formula:
{ [massa x (%)a] + [ massb x (%)b] + [massc x (%)c] + [ massdx (%)d] } ÷ 100
Weighted average mass of our sample of noodlium is: ________________ g
Questions
1. Is your weighted average mass consistent with the total sample mass?
2. Define isotope. Explain the differences between neon-19, neon-20, and neon-22.
3. Using the data provided, find the Atomic Masses of the following elements:
a.
b.
Isotope Atomic Mass
H-1
H–2
H–3
1.008amu
2.014amu
3.016amu
Percent
Abundance
99.985%
0.015%
Neglect
c.
d.
Isotope Atomic
Mass
Li - 6
6.015amu
Li – 7 7.016amu
e.
Isotope
Pb – 206
Pb - 207
Pb - 208
Percent
Abundance
7.68%
92.32%
Atomic
Mass
205.98amu
206.98amu
207.98amu
Percent
Abundance
x
x
100-2x
Isotope Atomic
Mass
Cl – 35 35.01amu
Cl – 37 37.013amu
Percent
Abundance
75.8%
24.2%
Isotope
Percent
Abundance
78.60%
10.11%
11.29%
Mg -24
Mg- 25
Mg- 26
Atomic
Mass
23.98amu
24.99amu
25.98amu