Ionic Bond Practice - sciencewithskinner
advertisement

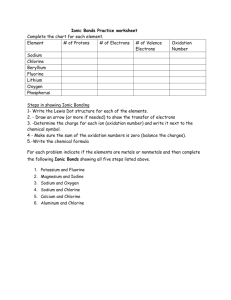
Name: ___________________________ Date: ____________ Period: ____ Ionic Bond Practice Background: - charged atoms called ions interact to form ionic bonds - ions are formed when electrons are transferred from one atom to another - this transfer of electrons occurs to stabilize atoms - an attractive force between these oppositely charged ions holds these atoms together - this force is called an ionic bond Practice: 1. Draw the electron shell diagram of the two atoms below, sodium (Na) and chlorine (Cl). 2. Transfer an electron from sodium to chlorine. Use an arrow to denote the transfer. 3. Draw the new ions (with their respective charges) and the compound that results from this ionic bond. + Na Cl NaCl (sodium chloride compound) 4. Are the valence shells of the ions of sodium and chlorine filled to capacity? 5. Are the atoms stable as a compound? Explain. 6. What is the charge of the sodium ion? Chlorine ion? Practice: 1. Draw the electron shell diagram of lithium and fluorine below. 2. Form an ionic compound (LiF – lithium fluoride) by transferring the electron(s). Use arrow(s) to denote the transfer. + Li F LiF Practice: 1. Draw the electron shell diagram of magnesium and two atoms of chlorine. 2. Form an ionic compound (MgCl2 – magnesium chloride) by transferring the electron(s). Use arrow(s) to denote the transfer. Mg Cl MgCl2 Cl