Ionic Bonds Practice Worksheet
advertisement

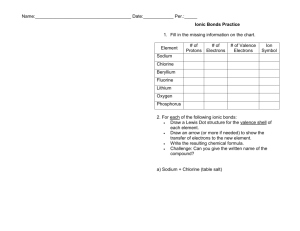
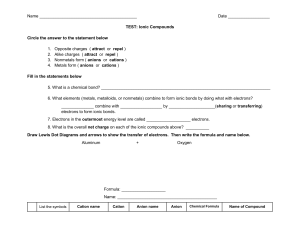
Ionic Bonds Practice worksheet Complete the chart for each element. Element # of Protons # of Electrons # of Valence Electrons Sodium Chlorine Beryllium Fluorine Lithium Oxygen Phosphorus Oxidation Number Steps in showing Ionic Bonding 1- Write the Lewis Dot structure for each of the elements. 2. - Draw an arrow (or more if needed) to show the transfer of electrons 3. -Determine the charge for each ion (oxidation number) and write it next to the chemical symbol. 4 - Make sure the sum of the oxidation numbers is zero (balance the charges). 5.-Write the chemical formula For each problem indicate if the elements are metals or nonmetals and then complete the following Ionic Bonds showing all five steps listed above. 1. Potassium and Fluorine 2. Magnesium and Iodine 3. Sodium and Oxygen 4. Sodium and Chlorine 5. Calcium and Chlorine 6. Aluminum and Chlorine