Ionic Bonding Worksheet: Chemthink.com
advertisement

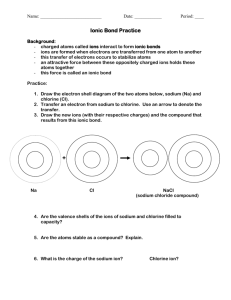
Chemthink.com – Ionic Bonding 1. Ionic Bonds are formed between __________. 2. Ionic Bonding involves ___________ of electrons 3. What happens when you move the two negatively charged ions near each other? 4. What happens when you move the oppositely charged ions near each other? 5. Opposite charges ___________, but same charges ________________. 6. In order to build an ionic compound that will stick together we need both a _________________________ and a ____________________________. 7. Atoms on the left side of the periodic table form ___________ ions by ______________ electrons. 8. Atoms on the right side of the periodic table form _______________ions by ________________ electrons. 9. What type (or types!) of elements are needed to form an ionic bond? 10. Briefly explain what happens between sodium and chlorine in the bonding scenario. What unusual circumstance does chlorine have that we have to be aware of to make this work correctly? 11. What happens when there are multiple sodium and chlorine ions? What is this called (when many ion pairs are attracted)? 12. For NaCl, the ratio of sodium ions to chloride ions is _____:_____. 13. For Calcium and Fluoride, the ratio is _____:_____, which can be reduced to _____:_____. Therefore the formula is written as _________________. 14. If you feel ready, try the questions. If not, go through the tutorial again. For the questions, you must get 10 questions correct before getting 3 wrong. If you do not do this, you must start over. You may take as many attempts as needed to get your 10 correct questions. Your progress is tracked on the right side of the screen. 15. Hint: some questions have more than one answer, so look carefully at all answer choices before hitting submit. 16. You do not need to send anything to me as long as you are registered and logged into my class and complete it by the due date. Chemthink collects the info online where I can access all results.