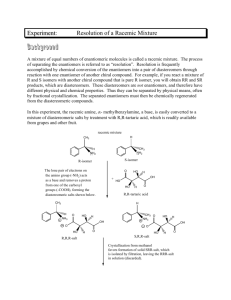
benzocaine

Outline of Experiment (in point form)
Esterification
1.
8mL of ethanol added to 0.85g (5mmol) of 4-nitrobenzoic acid and 1.2mL of conc.
H
2
SO
4
into a 50mL round bottom flask
2.
The mixture refluxed using a cold-water condenser.
3.
Mixture poured onto 2 hunks of ice (apx 12g) with 12mL of 10% NaOH
(aq)
.
4.
The precipitated ester suction filtered off, dried, weighed, and determined M.P.
Reduction
1.
95% EtOH (15ml) added to a solution of CaCl
2
(0.25g) in water (3ml) in a 50mL round bottom flask. Added ethyl 4-nitrobenzoate (0.60g, 3mmol) and zinc dust (6g)
2.
Refluxed for 1 hour
3.
Filtered by suction to remove zinc, without removing the filtrate, washed filter paper with ether (2x15mL)
4.
Washed the filtrate with brine (2x30mL) and extracted the brine with ether (2x15mL)
5.
Combine the ether extracts, washed with water (25mL), dried with magnesium sulfate
6.
Evaporated extracts to dryness.
7.
Dissolve solid in hot EtOH, add water until a cloudiness results. Allow to cool, then further in an ice bath. Filter and dry product
8.
Obtained melting point, and weight of ethyl 4-nitrobenzoate (benzocaine)
Results
Esterification
0.883g (5.28mmol) of 4-nitrobenzoic acid was used. The reflux was maintained at 1 drop per second until all the yellowish white acid reacted, approximately 20 minutes later.
When adding to the beaker, a white precipitate formed. All of the ice did not dissolve, so the flask was heated in a warm water bath until melting. The sample was oven dried for
5 minutes to leave 2.022g (10.4mmol) of still wet 4-nitrobenzoic acid. A melting point was determined (56-60
C). (lit. 56-58
C)
Reduction
Used 15mL EtOH, 0.273g CaCl
2
, 6.163g Zn dust, 0.641g (3.28mmol) Ethyl 4-
Nitrobenzoate (195.1744g/mol) and 3mL water. Refluxed for 60 minutes. Ether layers did not evaporate. A mysterious liquid resulted. Less than a milligram was recovered after lots of attempts to isolate a solid. A melting point of 98-100
C was determined for the mystery solid (lit. 89
C). No product was isolated.
0% yield.
Conclusion
This method of esterification seems to produce good yields, however the same cannot be said for the reduction using Zn/H
2
O, and I think it would be best to consider another reduction system, such as Fe/HCl, NaBH
3
or Al/Hg. The resulting chemical that was isolated was just a thin film of white gunk covering the filter paper. A melting point test was conducted which verified that it was not benzocaine. The outline of the work up looks correct and was performed as outlined. It seems that the nitro group never reduced to form the amine.